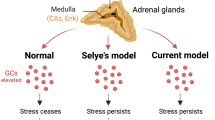
Abstract
In concert with neuropeptides and transmitters, the end products of the hypothalamus-pituitary-adrenal (HPA) axis, the glucocorticoid hormones cortisol and corticosterone (CORT), promote resilience: i.e., the ability to cope with threats, adversity, and trauma. To exert this protective action, CORT activates mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) that operate in a complementary manner -as an on/off switch- to coordinate circadian events, stress-coping, and adaptation. The evolutionary older limbic MR facilitates contextual memory retrieval and supports an on-switch in the selection of stress-coping styles at a low cost. The rise in circulating CORT concentration after stress subsequently activates a GR-mediated off-switch underlying recovery of homeostasis by providing the energy for restraining the primary stress reactions and promoting cognitive control over emotional reactivity. GR activation facilitates contextual memory storage of the experience to enable future stress-coping. Such complementary MR-GR-mediated actions involve rapid non-genomic and slower gene-mediated mechanisms; they are time-dependent, conditional, and sexually dimorphic, and depend on genetic background and prior experience. If coping fails, GR activation impairs cognitive control and promotes emotional arousal which eventually may compromise resilience. Such breakdown of resilience involves a transition to a chronic stress construct, where information processing is crashed; it leads to an imbalanced MR-GR switch and hence increased vulnerability. Novel MR-GR modulators are becoming available that may reset a dysregulated stress response system to reinstate the cognitive flexibility required for resilience.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Shorter E, Fink M. Endocrine psychiatry: solving the riddle of melancholia. Oxford University Press; 2010.
Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, et al. A specific laboratory test for the diagnosis of melancholia: standardization, validation, and clinical utility. Arch Gen Psychiatry. 1981;38:15–22.
Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N, et al. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression-a potential biomarker? Biol Psychiatry. 2007;62:47–54.
McEwen BS, Akil H. Revisiting the stress concept: implications for affective disorders. J Neurosci. 2020;40:12–21.
Selye H. STRESS - the physiology and pathology of exposure to stress. Acta Inc Montr. 1950;203:1025.
Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44.
Agorastos A, Chrousos GP. The neuroendocrinology of stress: the stress-related continuum of chronic disease development. Mol Psychiatry. 2022;27:502–13.
Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–81.
Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56.
Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66.
Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, Yehuda R. Resilience definitions, theory, and challenges: Interdisciplinary perspectives. Eur J Psychotraumatol. 2014;5.
McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–2.
Baker ME, Katsu Y. Evolution of the mineralocorticoid receptor. Vitam Horm. 2019;109:17–36.
Evans RM, Arriza JL. A molecular framework for the actions of glucocorticoid hormones in the nervous system. Neuron. 1989;2:1105–12.
Quinkler M, Meyer B, Bumke-Vogt C, Grossmann C, Gruber U, Oelkers W, et al. Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. Eur J Endocrinol. 2002;146:789–800.
Chapman K, Holmes M, Seckl J. 11-Hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93:1139–206.
Gomez-Sanchez EP, Gomez-Sanchez CE. 11β-hydroxysteroid dehydrogenases: a growing multi-tasking family. Mol Cell Endocrinol. 2021;526:111210.
Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11.
van Weert LTCM, Buurstede JC, Sips HCM, Mol IM, Puri T, Damsteegt R, et al. Mechanistic insights in NeuroD potentiation of mineralocorticoid receptor signaling. Int J Mol Sci. 2019;20:1575.
Meijer OC, Buurstede JC, Schaaf MJM. Corticosteroid receptors in the brain: transcriptional mechanisms for specificity and context-dependent effects. Cell Mol Neurobiol. 2019;39:539–49.
McEwen BS. Redefining neuroendocrinology: epigenetics of brain-body communication over the life course. Front Neuroendocrinol. 2018;49:8–30.
Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–7.
Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci 2005;102:19204–7.
Groeneweg FL, Karst H, de Kloet ER, Joëls M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol Cell Endocrinol. 2012;350:299–309.
Picard M, McEwen BS, Epel ES, Sandi C. An energetic view of stress: Focus on mitochondria. Front Neuroendocrinol. 2018;49:72–85.
Viho EMG, Buurstede JC, Mahfouz A, Koorneef LL, van Weert LTCM, Houtman R. et al. Corticosteroid action in the brain: the potential of selective receptor modulation. Neuroendocrinology. 2019;109:266–76.
de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75.
de Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301.
Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501.
Joëls M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012;64:901–38.
Lightman SL, Birnie MT, Conway-Campbell BL. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr Rev. 2020;41:470–90.
Ratka A, Sutanto W, Bloemers M, de Kloet ER. On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology. 1989;50:117–23.
Young EA. The role of mineralocorticoid receptors in hypothalamic-pituitary-adrenal axis regulation in humans. J Clin Endocrinol Metab. 1998;83:3339–45.
van Haarst AD, Oitzl MS, Workel JO, de Kloet ER. Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology. 1996;137:4935–43.
Dallman MF, Levin N, Cascio CS, Akana SF, Jacobson L, Kuhn RW. Pharmacological evidence that the inhibition of diurnal adrenocorticotropin secretion by corticosteroids is mediated via type I corticosterone-preferring receptors. Endocrinology. 1989;124:2844–50.
Sarabdjitsingh RA, Isenia S, Polman A, Mijalkovic J, Lachize S, Datson N, et al. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology. 2010;151:1177–86.
Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, et al. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol. 2010;22:1093–1100.
Sarabdjitsingh RA, Jezequel J, Pasricha N, Mikasova L, Kerkhofs A, Karst H, et al. Ultradian corticosterone pulses balance glutamatergic transmission and synaptic plasticity. Proc Natl Acad Sci USA 2014;111:14265–70.
Groch S, Wilhelm I, Lange T, Born J. Differential contribution of mineralocorticoid and glucocorticoid receptors to memory formation during sleep. Psychoneuroendocrinology. 2013;38:2962–72.
Rimmele U, Besedovsky L, Lange T, Born J. Blocking mineralocorticoid receptors impairs, blocking glucocorticoid receptors enhances memory retrieval in humans. Neuropsychopharmacology. 2013;38:884–94.
Kelemen E, Bahrendt M, Born J, Inostroza M. Hippocampal corticosterone impairs memory consolidation during sleep but improves consolidation in the wake state. Hippocampus. 2014;24:510–5.
Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan W-B. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705.
Henkin RI, Daly RL. Auditory detection and perception in normal man and in patients with adrenal cortical insufficiency: effect of adrenal cortical steroids. J Clin Investig. 1968;47:1269–80.
Obleser J, Kreitewolf J, Vielhauer R, Lindner F, David C, Oster H, et al. Circadian fluctuations in glucocorticoid level predict perceptual discrimination sensitivity. IScience. 2021;24:102345.
Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409.
van den Berg DTWM, de Kloet ER, van Dijken HH, de Jong W, de Kloet ER. Differential central effects of mineralocorticoid and glucocorticoid agonists and antagonists on blood pressure. Endocrinology. 1990;126:118–24.
Cornelisse S, Joëls M, Smeets T. A randomized trial on mineralocorticoid receptor blockade in men: Effects on stress responses, selective attention, and memory. Neuropsychopharmacology. 2011;36:2720–8.
Oitzl MS, Fluttert M, Ron de Kloet E. The effect of corticosterone on reactivity to spatial novelty is mediated by central mineralocorticosteroid receptors. Eur J Neurosci. 1994;6:1072–9.
Korte SM, de Boer SF, de Kloet ER, Bohus B. Anxiolytic-like effects of selective mineralocorticoid and glucocorticoid antagonists on fear-enhanced behavior in the elevated plus-maze. Psychoneuroendocrinology. 1995;20:385–94.
Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, et al. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci USA 2006;103:195–200.
Kruk MR, Haller J, Meelis W, de Kloet ER. Mineralocorticoid receptor blockade during a rat’s first violent encounter inhibits its subsequent propensity for violence. Behav Neurosci. 2013;127:505–14.
McCann KE, Lustberg DJ, Shaughnessy EK, Carstens KE, Farris S, Alexander GM, et al. Novel role for mineralocorticoid receptors in control of a neuronal phenotype. Mol Psychiatry. 2021;26:350–64.
Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci USA 2007;104:4688–93.
Mitra R, Ferguson D, Sapolsky RM. Mineralocorticoid receptor overexpression in basolateral amygdala reduces corticosterone secretion and anxiety. Biol Psychiatry. 2009;66:686–90.
Arnett MG, Muglia LM, Laryea G, Muglia LJ. Genetic approaches to hypothalamic-pituitary-adrenal axis regulation. Neuropsychopharmacology. 2016;41:245–60.
Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM. Genetic selection for coping style predicts stressor susceptibility. J Neuroendocrinol. 2003;15:256–67.
Steimer T, Driscoll P. Divergent stress responses and coping styles in psychogenetically selected roman high-(RHA) and low-(RLA) avoidance rats: behavioural, neuroendocrine and developmental aspects. Stress. 2003;6:87–100.
Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992;106:62–71.
Souza RR, Dal Bó S, de Kloet ER, Oitzl MS, Carobrez AP. Paradoxical mineralocorticoid receptor-mediated effect in fear memory encoding and expression of rats submitted to an olfactory fear conditioning task. Neuropharmacology. 2014;79:201–11.
Harris AP, Holmes MC, de Kloet ER, Chapman KE, Seckl JR. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology. 2013;38:648–58.
Schwabe L, Schächinger H, de Kloet ER, Oitzl MS. Corticosteroids operate as a switch between memory systems. J Cogn Neurosci. 2010;22:1362–72.
Arp JM, ter Horst JP, Kanatsou S, Fernández G, Joëls M, Krugers HJ, et al. Mineralocorticoid receptors guide spatial and stimulus-response learning in mice. PLoS ONE. 2014;9:e86236.
Schwabe L, Tegenthoff M, Höffken O, Wolf OT. Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biol Psychiatry. 2013;74:801–8.
Vogel S, Fernández G, Joëls M, Schwabe L. Cognitive adaptation under stress: a case for the mineralocorticoid receptor. Trends Cogn Sci. 2016;20:192–203.
Wirz L, Reuter M, Wacker J, Felten A, Schwabe L. A haplotype associated with enhanced mineralocorticoid receptor expression facilitates the stress-induced shift from ‘cognitive’ to ‘habit’ learning. ENeuro. 2017;4:ENEURO.0359–17.2017.
van Leeuwen N, Bellingrath S, de Kloet ER, Zitman FG, DeRijk RH, Kudielka BM, et al. Human mineralocorticoid receptor (MR) gene haplotypes modulate MR expression and transactivation: implication for the stress response. Psychoneuroendocrinology. 2011;36:699–709.
Klok MD, Giltay EJ, van der Does AJW, Geleijnse JM, Antypa N, Penninx BWJH, et al. A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Transl Psychiatry. 2011;1:e62.
Hamstra DA, de Kloet ER, Quataert I, Jansen M, van der Does W. Mineralocorticoid receptor haplotype, estradiol, progesterone and emotional information processing. Psychoneuroendocrinology. 2017;76:162–73.
Vinkers CH, Joëls M, Milaneschi Y, Gerritsen L, Kahn RS, Penninx BWJH, et al. Mineralocorticoid receptor haplotypes sex-dependently moderate depression susceptibility following childhood maltreatment. Psychoneuroendocrinology. 2015;54:90–102.
Gerritsen L, Milaneschi Y, Vinkers CH, van Hemert AM, van Velzen L, Schmaal L, et al. HPA axis genes, and their interaction with childhood maltreatment, are related to cortisol levels and stress-related phenotypes. Neuropsychopharmacology. 2017;42:2446–55.
Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am J Psychiatry. 2012;169:515–22.
Klok MD, Alt SR, Irurzun Lafitte AJM, Turner JD, Lakke EAJF, Huitinga I, et al. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J Psychiatr Res. 2011;45:871–8.
Medina A, Seasholtz AF, Sharma V, Burke S, Bunney W, Myers RM, et al. Glucocorticoid and mineralocorticoid receptor expression in the human hippocampus in major depressive disorder. J Psychiatr Res. 2013;47:307–14.
de Kloet ER, Otte C, Kumsta R, Kok L, Hillegers MHJ, Hasselmann H, et al. Stress and depression: a crucial role of the mineralocorticoid receptor. J Neuroendocrinol. 2016;28.
Kumsta R, Kliegel D, Linden M, DeRijk R, de Kloet ER. Genetic variation of the mineralocorticoid receptor gene (MR, NR3C2) is associated with a conceptual endophenotype of ‘CRF-hypoactivity’. Psychoneuroendocrinology. 2019;105:79–85.
de Kloet ER, de Kock S, Schild V, Veldhuis HD, Antiglucocorticoid RU. 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary adrenal axis at different brain sites. Neuroendocrinology. 1988;47:109–15.
Oitzl MS, Reichardt HM, Joëls M, de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc Natl Acad Sci USA. 2001;98:12790–5.
Bahtiyar S, Gulmez Karaca K. Henckens MJAG, Roozendaal B. Norepinephrine and glucocorticoid effects on the brain mechanisms underlying memory accuracy and generalization. Mol Cell Neurosci. 2020;108:103537.
Barsegyan A, Mirone G, Ronzoni G, Guo C, Song Q, van Kuppeveld D, et al. Glucocorticoid enhancement of recognition memory via basolateral amygdala-driven facilitation of prelimbic cortex interactions. Proc Natl Acad Sci USA. 2019;116:7077–82.
Karst H, Berger S, Erdmann G, Schütz G, Joëls M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA. 2010;107:14449–54.
Pacák K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–48.
Herman JP, Nawreen N, Smail MA, Cotella EM. Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress. 2020;23:617–32.
Bonapersona V, Schuler H, Damsteegt R, Adolfs Y, Pasterkamp RJ, van den Heuvel MP, et al. The mouse brain after foot shock in four dimensions: temporal dynamics at a single-cell resolution. Proc Natl Acad Sci USA. 2022;119:e2114002119.
Gutierrez-Mecinas M, Trollope AF, Collins A, Morfett H, Hesketh SA, Kersante F, et al. Long-lasting behavioral responses to stress involve a direct interaction of glucocorticoid receptors with ERK1/2-MSK1-Elk-1 signaling. Proc Natl Acad Sci USA 2011;108:13806–11.
Saunderson EA, Spiers H, Mifsud KR, Gutierrez-Mecinas M, Trollope AF, Shaikh A, et al. Stress-induced gene expression and behavior are controlled by DNA methylation and methyl donor availability in the dentate gyrus. Proc Natl Acad Sci USA 2016;113:4830–5.
Lesuis SL, Brosens N, Immerzeel N, van der Loo RJ, Mitrić M, Bielefeld P, et al. Glucocorticoids promote fear generalization by increasing the size of a dentate gyrus engram cell population. Biol Psychiatry. 2021;90:494–504.
Bouarab C, Roullot-Lacarrière V, Vallée M, le Roux A, Guette C, Mennesson M, et al. PAI-1 protein is a key molecular effector in the transition from normal to PTSD-like fear memory. Mol Psychiatry. 2021;26:4968–81.
Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA. 2005;102:473–8.
Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA. 2008;105:12004–9.
Solomon MB, Loftspring M, de Kloet AD, Ghosal S, Jankord R, Flak JN, et al. Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice. Endocrinology. 2015;156:2843–53.
Hartmann J, Dedic N, Pöhlmann ML, Häusl A, Karst H, Engelhardt C, et al. Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol Psychiatry. 2017;22:466–75.
Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41.
Matosin N, Halldorsdottir T, Binder EB. Understanding the molecular mechanisms underpinning gene by environment interactions in psychiatric disorders: the FKBP5 Model. Biol Psychiatry. 2018;83:821–30.
de Quervain D, Wolf OT, Roozendaal B. Glucocorticoid-induced enhancement of extinction-from animal models to clinical trials. Psychopharmacology. 2019;236:183–99.
Pitman RK, Milad MR, Igoe SA, Vangel MG, Orr SP, Tsareva A, et al. Systemic mifepristone blocks reconsolidation of cue-conditioned fear; propranolol prevents this effect. Behav Neurosci. 2011;125:632–8.
Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, van Manen JA, et al. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology. 2015;51:589–97.
de Kloet ER, de Kloet SF, de Kloet CS, de Kloet AD. Top-down and bottom-up control of stress-coping. J Neuroendocrinol. 2019;31:e12675.
Cai W-H. Postreactivation glucocorticoids impair recall of established fear memory. J Neurosci. 2006;26:9560–6.
Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: Interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21:796–809.
Carmi L, Zohar J, Weissman T, Juven-Wetzler A, Bierer L, Yehuda R, et al. Hydrocortisone in the emergency department: a prospective, double-blind, randomized, controlled posttraumatic stress disorder study. Hydrocortisone during golden hours. CNS Spectr. 2022; June 9:1–7.
Sousa N. The dynamics of the stress neuromatrix. Mol Psychiatry. 2016;21:302–12.
McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress. 2019;3:2470547019833647.
Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351–3.
Kim JS, Iremonger KJ. Temporally tuned corticosteroid feedback regulation of the stress axis. Trends Endocrinol Metab. 2019;30:783–92.
Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69.
Daskalakis NP, Cohen H, Cai G, Buxbaum JD, Yehuda R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proc Natl Acad Sci USA 2014;111:13529–34.
de Voogd LD, Kampen RA, Kaldewaij R, Zhang W, Hashemi MM, Koch SBJ, et al. Trauma-induced human glucocorticoid receptor expression increases predict subsequent HPA-axis blunting in a prospective longitudinal design. Psychoneuroendocrinology. 2022;146.
McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23.
Magalhães R, Barrière DA, Novais A, Marques F, Marques P, Cerqueira J, et al. The dynamics of stress: a longitudinal MRI study of rat brain structure and connectome. Mol Psychiatry. 2018;23:1998–2006.
Weger M, Alpern D, Cherix A, Ghosal S, Grosse J, Russeil J, et al. Mitochondrial gene signature in the prefrontal cortex for differential susceptibility to chronic stress. Sci Rep. 2020;10:18308.
Hunter RG, Seligsohn M, Rubin TG, Griffiths BB, Ozdemir Y, Pfaff DW, et al. Stress and corticosteroids regulate rat hippocampal mitochondrial DNA gene expression via the glucocorticoid receptor. Proc Natl Acad Sci USA. 2016;113:9099–104.
Szeszko PR, Lehrner A, Yehuda R. Glucocorticoids and hippocampal structure and function in PTSD. Harv Rev Psychiatry. 2018;26:142–57.
Brocca ME, Pietranera L, de Kloet ER, de Nicola AF. Mineralocorticoid receptors, neuroinflammation and hypertensive encephalopathy. Cell Mol Neurobiol. 2019;39:483–92.
Datson NA, van den Oever JME, Korobko OB, Magarinos AM, de Kloet ER, McEwen BS. Previous history of chronic stress changes the transcriptional response to glucocorticoid challenge in the dentate gyrus region of the male rat hippocampus. Endocrinology. 2013;154:3261–72.
Gray JD, Rubin TG, Hunter RG, McEwen BS. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry. 2014;19:1171–8.
Henckens MJAG, van der Marel K, van der Toorn A, Pillai AG, Fernández G, Dijkhuizen RM, et al. Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage. 2015;105:312–22.
Hermans EJ, Henckens MJ, Joels M, Fernandez G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304–14.
Schwabe L, Hermans EJ, Joëls M, Roozendaal B. Mechanisms of memory under stress. Neuron 2022;110:1450–67.
Berretz G, Packheiser J, Kumsta R, Wolf OT, Ocklenburg S. The brain under stress-a systematic review and activation likelihood estimation meta-analysis of changes in BOLD signal associated with acute stress exposure. Neurosci Biobehav Rev. 2021;124:89–99.
Szeszko PR, Yehuda R. Magnetic resonance imaging predictors of psychotherapy treatment response in post-traumatic stress disorder: A role for the salience network. Psychiatry Res. 2019;277:52–57.
Zhang W, Kaldewaij R, Hashemi MM, Koch SBJ, Smit A, van Ast VA, et al. Acute-stress-induced change in salience network coupling prospectively predicts post-trauma symptom development. Transl Psychiatry. 2022;12:63.
Ventura R, Cabib S, Babicola L, Andolina D, di Segni M, Orsini C. Interactions between experience, genotype and sex in the development of individual coping strategies. Front Behav Neurosci. 2021;15:785739.
Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–78.
Johnson SB, Lingg RT, Skog TD, Hinz DC, Romig-Martin SA, Viau V, et al. Activity in a prefrontal-periaqueductal gray circuit overcomes behavioral and endocrine features of the passive coping stress response. Proc Natl Acad Sci USA. 2022;119:e2210783119.
Giachero M, Pavesi E, Calfa G, Motta SC, Canteras NS, Molina VA, et al. Inactivation of the dorsolateral periaqueductal gray matter impairs the promoting influence of stress on fear memory during retrieval. Brain Struct Funct. 2019;224:3117–32.
Radley JJ, Johnson SB. Anteroventral bed nuclei of the stria terminalis neurocircuitry: Towards an integration of HPA axis modulation with coping behaviors - Curt Richter Award Paper 2017. Psychoneuroendocrinology. 2018;89:239–49.
Johnson SB, Emmons EB, Lingg RT, Anderson RM, Romig-Martin SA, Lalumiere RT, et al. Prefrontal-bed nucleus circuit modulation of a passive coping response set. J Neurosci. 2019;39:1405–19.
Lingg RT, Johnson SB, Emmons EB, Anderson RM, Romig-Martin SA, Narayanan NS, et al. Bed nuclei of the stria terminalis modulate memory consolidation via glucocorticoid-dependent and -independent circuits. Proc Natl Acad Sci USA. 2020;117:8104–14.
Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–5.
Cabib S, Latagliata C, Orsini C. Role of stress-related dopamine transmission in building and maintaining a protective cognitive reserve. Brain Sci. 2022;12:246.
Maier SF, Seligman MEP. Learned helplessness at fifty: insights from neuroscience. Psychol Rev. 2016;123:349–67.
McKlveen JM, Moloney RD, Scheimann JR, Myers B, Herman JP. ‘Braking’ the prefrontal cortex: the role of glucocorticoids and interneurons in stress adaptation and pathology. Biol Psychiatry. 2019;86:669–81.
Pace SA, Christensen C, Schackmuth MK, Wallace T, McKlveen JM, Beischel W, et al. Infralimbic cortical glutamate output is necessary for the neural and behavioral consequences of chronic stress. Neurobiol Stress. 2020;13:100274.
Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, Spencer RL. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J Neuroendocrinol. 2000;12:1034–42.
Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147:4917–30.
Tzanoulinou S, Gantelet E, Sandi C, Márquez C. Programming effects of peripubertal stress on spatial learning. Neurobiol Stress. 2020;13:100282.
Papilloud A, Veenit V, Tzanoulinou S, Riccio O, Zanoletti O, Guillot de Suduiraut I, et al. Peripubertal stress-induced heightened aggression: modulation of the glucocorticoid receptor in the central amygdala and normalization by mifepristone treatment. Neuropsychopharmacology. 2019;44:674–82.
Herbert J. Cortisol and depression: three questions for psychiatry. Psychol Med. 2013;43:449–69.
Mayer JL, Klumpers L, Maslam S, de Kloet ER, Joëls M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J Neuroendocrinol. 2006;18:629–31.
Oomen CA, Mayer JL, de Kloet ER, Joëls M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci. 2007;26:3395–401.
Hu P, Oomen C, van Dam A-M, Wester J, Zhou J-N, Joëls M, et al. A single-day treatment with mifepristone is sufficient to normalize chronic glucocorticoid induced suppression of hippocampal cell proliferation. PLoS ONE. 2012;7:e46224.
Kroon J, Viho EMG, Gentenaar M, Koorneef LL, van Kooten C, Rensen PCN, et al. The development of novel glucocorticoid receptor antagonists: From rational chemical design to therapeutic efficacy in metabolic disease models. Pharm Res. 2021;168:105588.
Baglietto-Vargas D, Medeiros R, Martinez-Coria H, LaFerla FM, Green KN. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol Psychiatry. 2013;74:357–66.
Meyer M, Kruse MS, Garay L, Lima A, Roig P, Hunt H, et al. Long-term effects of the glucocorticoid receptor modulator CORT113176 in murine motoneuron degeneration. Brain Res. 2020;1727:146551.
Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–71.
McGinn MA, Tunstall BJ, Schlosburg JE, Gregory-Flores A, George O, de Guglielmo G, et al. Glucocorticoid receptor modulators decrease alcohol self-administration in male rats. Neuropharmacology. 2021;188:108510.
Block T, Petrides G, Kushner H, Kalin N, Belanoff J, Schatzberg A. Mifepristone plasma level and glucocorticoid receptor antagonism associated with response in patients with psychotic depression. J Clin Psychopharmacol. 2017;37:505–11.
Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–46.
Levine S. Infantile experience and resistance to physiological stress. Science. 1979;1957:405.
Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1979;1997:1659–62.
Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–45.
Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joëls M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem. 2009;92:292–300.
Peña CJ, Nestler EJ, Bagot RC. Environmental programming of susceptibility and resilience to stress in adulthood in male mice. Front Behav Neurosci. 2019;13:40.
Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav. 2012;106:691–700.
Bonapersona V, Kentrop J, van Lissa CJ, van der Veen R, Joëls M, Sarabdjitsingh RA. The behavioral phenotype of early life adversity: A 3-level meta-analysis of rodent studies. Neurosci Biobehav Rev. 2019;102:299–307.
Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, et al. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress. 2017;20:421–48.
van Oers HJJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci. 1998;18:10171–9.
Daskalakis NP, Claessens SEF, Laboyrie JJL, Enthoven L, Oitzl MS, Champagne DL, et al. The newborn rat’s stress system readily habituates to repeated and prolonged maternal separation, while continuing to respond to stressors in context dependent fashion. Horm Behav. 2011;60:165–76.
Boyce WT, Levitt P, Martinez FD, McEwen BS, Shonkoff JP. Genes, environments, and time: The biology of adversity and resilience. Pediatrics. 2021;147:e20201651.
Arp JM, ter Horst JP, Loi M, den Blaauwen J, Bangert E, Fernández G, et al. Blocking glucocorticoid receptors at adolescent age prevents enhanced freezing between repeated cue-exposures after conditioned fear in adult mice raised under chronic early life stress. Neurobiol Learn Mem. 2016;133:30–38.
Loi M, Sarabdjitsingh RA, Tsouli A, Trinh S, Arp M, Krugers HJ, et al. Transient prepubertal mifepristone treatment normalizes deficits in contextual memory and neuronal activity of adult male rats exposed to maternal deprivation. ENeuro. 2017;4:ENEURO.0253–17.2017.
Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ, WEIGHT IN. Infancy and death from ischaemic heart disease. Lancet. 1989;334:577–80.
Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26:6737–48.
Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Dev Psychobiol. 2010;52:651–60.
Daskalakis NP, Diamantopoulou A, Claessens SEF, Remmers E, Tjälve M, Oitzl MS, et al. Early experience of a novel-environment in isolation primes a fearful phenotype characterized by persistent amygdala activation. Psychoneuroendocrinology. 2014;39:39–57.
Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA. 2012;109:E1312–E1319.
Soe NN, Wen DJ, Poh JS, Chong YS, Broekman BF, Chen H, et al. Perinatal maternal depressive symptoms alter amygdala functional connectivity in girls. Hum Brain Mapp. 2018;39:680–90.
Graham AM, Rasmussen JM, Entringer S, ben Ward E, Rudolph MD, Gilmore JH, et al. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol Psychiatry. 2019;85:172–81.
Turecki G, Meaney MJ. Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol Psychiatry. 2016;79:87–96.
Galbally M, Watson SJ, van IJzendoorn M, Saffery R, Ryan J, de Kloet ER, et al. The role of glucocorticoid and mineralocorticoid receptor DNA methylation in antenatal depression and infant stress regulation. Psychoneuroendocrinology. 2020;115:104611.
Jahnke JR, Terán E, Murgueitio F, Cabrera H, Thompson AL. Maternal stress, placental 11β-hydroxysteroid dehydrogenase type 2, and infant HPA axis development in humans: psychosocial and physiological pathways. Placenta. 2021;104:179–87.
Galbally M, Watson SJ, Lappas M, de Kloet ER, Wyrwoll CS, Mark PJ, et al. Exploring sex differences in fetal programming for childhood emotional disorders. Psychoneuroendocrinology. 2022;141:105764.
Heck AL, Handa RJ. Sex differences in the hypothalamic–pituitary–adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44:45–58.
Droste SK, de Groote L, Lightman SL, Reul JMHM, Linthorst ACE. The ultradian and circadian rhythms of free corticosterone in the brain are not affected by gender: an in vivo microdialysis study in Wistar rats. J Neuroendocrinol. 2009;21:132–40.
Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–21.
Kroon J, Pereira AM, Meijer OC. Glucocorticoid sexual dimorphism in metabolism: dissecting the role of sex hormones. Trends Endocrinol Metab. 2020;31:357–67.
Bale TL. The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues Clin Neurosci. 2016;18:459–64.
Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–19.
Wellman CL, Bangasser DA, Bollinger JL, Coutellier L, Logrip ML, Moench KM, et al. Sex differences in risk and resilience: stress effects on the neural substrates of emotion and motivation. J Neurosci. 2018;38:9423–32.
Moisan M-P. Sexual dimorphism in glucocorticoid stress response. Int J Mol Sci. 2021;22:3139.
ter Horst JP, Kentrop J, Arp M, Hubens CJ, de Kloet ER, Oitzl MS. Spatial learning of female mice: a role of the mineralocorticoid receptor during stress and the estrous cycle. Front Behav Neurosci. 2013;7:1–10.
ter Horst JP, van der Mark M, Kentrop J, Arp M, van der Veen R, de Kloet ER, et al. Deletion of the forebrain mineralocorticoid receptor impairs social discrimination and decision-making in male, but not in female mice. Front Behav Neurosci. 2014;8:26.
Scheimann JR, Moloney RD, Mahbod P, Morano RL, Fitzgerald M, Hoskins O, et al. Conditional deletion of glucocorticoid receptors in rat brain results in sex-specific deficits in fear and coping behaviors. Elife. 2019;8:e44672.
Luine V, Gomez J, Beck K, Bowman R. Sex differences in chronic stress effects on cognition in rodents. Pharm Biochem Behav. 2017;152:13–19.
ter Horst JP, Kentrop J, de Kloet ER, Oitzl MS. Stress and estrous cycle affect strategy but not performance of female C57BL/6J mice. Behavioural Brain Res. 2013;241:92–95.
Kokras N, Krokida S, Varoudaki TZ, Dalla C. Do corticosterone levels predict female depressive‐like behavior in rodents? J Neurosci Res. 2021;99:324–31.
McEwen BS. Hormones and behavior and the integration of brain-body science. Horm Behav. 2020;119:104619.
McCarthy MM. A new view of sexual differentiation of mammalian brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020;206:369–78.
Duma D, Collins JB, Chou JW, Cidlowski JA. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci Signal. 2010;3:ra74.
Tejos-Bravo M, Oakley RH, Whirledge SD, Corrales WA, Silva JP, García-Rojo G, et al. Deletion of hippocampal Glucocorticoid receptors unveils sex-biased microRNA expression and neuronal morphology alterations in mice. Neurobiol Stress. 2021;14:100306.
Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–32.
Stephens MAC, Mahon PB, McCaul ME, Wand GS. Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 2016;66:47–55.
Zorn JV, Schür RR, Boks MP, Kahn RS, Joëls M, Vinkers CH. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology. 2017;77:25–36.
Hartmann J, Bajaj T, Klengel C, Chatzinakos C, Ebert T, Dedic N, et al. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 2021;35:109185.
Mifsud KR, Kennedy CLM, Salatino S, Sharma E, Price EM, Haque SN, et al. Distinct regulation of hippocampal neuroplasticity and ciliary genes by corticosteroid receptors. Nat Commun. 2021;12:4737.
Oakley RH, Whirledge SD, Petrillo MG, Riddick NV, Xu X, Moy SS, et al. Combinatorial actions of glucocorticoid and mineralocorticoid stress hormone receptors are required for preventing neurodegeneration of the mouse hippocampus. Neurobiol Stress. 2021;15:100369.
Joëls M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27:244–50.
Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007;2007:60803.
Penner-Goeke S, Binder EB. Epigenetics and depression. Dialogues Clin Neurosci. 2019;21:397–405.
Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45.
Kwako LE, Koob GF. Neuroclinical framework for the role of stress in addiction. Chronic Stress. 2017;1:247054701769814.
Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Investig. 2015;125:3193–7.
Farokhnia M, Rentsch CT, Chuong V, McGinn MA, Elvig SK, Douglass EA, et al. Spironolactone as a potential new pharmacotherapy for alcohol use disorder: convergent evidence from rodent and human studies. Mol Psychiatry. 2022;27:1–11.
Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–5.
Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, et al. Treatment resistant depression: a multi-scale, systems biology approach. Neurosci Biobehav Rev. 2018;84:272–88.
Provençal N, Arloth J, Cattaneo A, Anacker C, Cattane N, Wiechmann T, et al. Glucocorticoid exposure during hippocampal neurogenesis primes future stress response by inducing changes in DNA methylation. Proc Natl Acad Sci USA. 2020;117:23280–85.
Suarez A, Lahti J, Lahti-Pulkkinen M, Girchenko P, Czamara D, Arloth J, et al. A polyepigenetic glucocorticoid exposure score at birth and childhood mental and behavioral disorders. Neurobiol Stress. 2020;13:100275.
Chatzinakos C, Georgiadis F, Daskalakis NP. GWAS meets transcriptomics: from genetic letters to transcriptomic words of neuropsychiatric risk. Neuropsychopharmacology. 2021;46:255–6.
Dalvie S, Chatzinakos C, Al Zoubi O, Georgiadis F, Lancashire L, Daskalakis NP. From genetics to systems biology of stress-related mental disorders. Neurobiol Stress. 2021;15:100393.
Daskalakis NP, Meijer OC, de Kloet ER. Mineralocorticoid receptor and glucocorticoid receptor work alone and together in cell-type-specific manner: Implications for resilience prediction and targeted therapy. Neurobiol Stress. 2022;18:100455.
Penner-Goeke S, Bothe M, Kappelmann N, Kreitmaier P, Kaya E, Pöhlchen D, et al. Assessment of glucocorticoid-induced enhancer activity of eSNP regions using STARR-seq reveals novel molecular mechanisms in psychiatric disorders. 2022. https://www.medrxiv.org/content/10.1101/2022.05.18.22275090v1.
Zalachoras I, Verhoeve SL, Toonen LJ, van Weert LTCM, van Vlodrop AM, Mol IM, et al. Isoform switching of steroid receptor co-activator-1 attenuates glucocorticoid-induced anxiogenic amygdala CRH expression. Mol Psychiatry. 2016;21:1733–9.
Fuller PJ, Yang J, Young MJ. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: coregulators as mediators of mineralocorticoid receptor signalling diversity. J Endocrinol. 2017;234:T23–T34.
Zalachoras I, Houtman R, Atucha E, Devos R, Tijssen AMI, Hu P, et al. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc Natl Acad Sci USA. 2013;110:7910–5.
Atucha E, Zalachoras I, van den Heuvel JK, van Weert LTCM, Melchers D, Mol IM, et al. A mixed glucocorticoid/mineralocorticoid selective modulator with dominant antagonism in the male rat brain. Endocrinology. 2015;156:4105–14.
Warris LT, van den Heuvel-Eibrink MM, Aarsen FK, Pluijm SMF, Bierings MB, van Bos Cden, et al. Hydrocortisone as an intervention for dexamethasone-induced adverse effects in pediatric patients with acute lymphoblastic leukemia: results of a double-blind, randomized controlled trial. J Clin Oncol. 2016;34:2287–93.
Jeanneteau F, Meijer OC, Moisan M. Structural basis of glucocorticoid receptor signaling bias. J Neuroendocrinol. 2022;e13203.
Dalm S, Karssen AM, Meijer OC, Belanoff JK, de Kloet ER. Resetting the stress system with a mifepristone challenge. Cell Mol Neurobiol. 2019;39:503–22.
Hellhammer D, Meinlschmidt G, Pruessner JC. Conceptual endophenotypes: a strategy to advance the impact of psychoneuroendocrinology in precision medicine. Psychoneuroendocrinology. 2018;89:147–60.
Fava GA, McEwen BS, Guidi J, Gostoli S, Offidani E, Sonino N. Clinical characterization of allostatic overload. Psychoneuroendocrinology. 2019;108:94–101.
Chen C, Nakagawa S, An Y, Ito K, Kitaichi Y, Kusumi I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front Neuroendocrinol. 2017;44:83–102.
Guidi J, Fava GA. Sequential combination of pharmacotherapy and psychotherapy in major depressive disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78:261–9.
Harris C, Weiss GL, Di S, Tasker JG. Cell signaling dependence of rapid glucocorticoid-induced endocannabinoid synthesis in hypothalamic neuroendocrine cells. Neurobiol Stress. 2019;10:100158.
Nixon M, Mackenzie SD, Taylor AI, Homer NZM, Livingstone DE, Mouras R, et al. ABCC1 confers tissue-specific sensitivity to cortisol versus corticosterone: A rationale for safer glucocorticoid replacement therapy. Sci Transl Med. 2016;8:352ra109.
Karssen AM, Meijer OC, Berry A, Sanjuan Piñol R, de Kloet ER. Low doses of dexamethasone can produce a hypocorticosteroid state in the brain. Endocrinology. 2005;146:5587–95.
Gasparini S, Resch JM, Narayan SV, Peltekian L, Iverson GN, Karthik S, et al. Aldosterone-sensitive HSD2 neurons in mice. Brain Struct Funct. 2019;224:387–417.
Künzel H. Psychopathological symptoms in patients with primary hyperaldosteronism - possible pathways. Horm Metab Res. 2012;44:202–7.
Hlavacova N, Jezova D. Chronic treatment with the mineralocorticoid hormone aldosterone results in increased anxiety-like behavior. Horm Behav. 2008;54:90–97.
Mifsud KR, Reul JMHM. Acute stress enhances heterodimerization and binding of corticosteroid receptors at glucocorticoid target genes in the hippocampus. Proc Natl Acad Sci USA. 2016;113:11336–41.
Foley P, Kirschbaum C. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci Biobehav Rev. 2010;35:91–96.
Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. 2017;77:261–74.
de Kloet R, Wallach G, McEwen BS. Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology. 1975;96:598–609.
Moore SR, Halldorsdottir T, Martins J, Lucae S, Müller-Myhsok B, Müller NS, et al. Sex differences in the genetic regulation of the blood transcriptome response to glucocorticoid receptor activation. Transl Psychiatry. 2021;11:632.
Cruceanu C, Dony L, Krontira AC, Fischer DS, Roeh S, di Giaimo R, et al. Cell-type-specific impact of glucocorticoid receptor activation on the developing brain: a cerebral organoid study. Am J Psychiatry. 2022;179:375–87.
Carrillo-Roa T, Labermaier C, Weber P, Herzog DP, Lareau C, Santarelli S, et al. Common genes associated with antidepressant response in mouse and man identify key role of glucocorticoid receptor sensitivity. PLoS Biol. 2017;15:e2002690.
Meijer OC, de Lange ECM, Breimer DD, de Boer AG, Workel JO, de Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology. 1998;139:1789–93.
Judd LL, Schettler PJ, Brown ES, Wolkowitz OM, Sternberg EM, Bender BG, et al. Adverse consequences of glucocorticoid medication: psychological, cognitive, and behavioral effects. Am J Psychiatry. 2014;171:1045–51.
Fitzsimons CP, van Hooijdonk LWA, Schouten M, Zalachoras I, Brinks V, Zheng T, et al. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol Psychiatry. 2013;18:993–1005.
Häusl AS, Brix LM, Hartmann J, Pöhlmann ML, Lopez J-P, Menegaz D, et al. The co-chaperone Fkbp5 shapes the acute stress response in the paraventricular nucleus of the hypothalamus of male mice. Mol Psychiatry. 2021;26:3060–76.
Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–8.
Karst H, Joëls M. Severe stress hormone conditions cause an extended window of excitability in the mouse basolateral amygdala. Neuropharmacology. 2016;110:175–80.
den Boon FS, de Vries T, Baelde M, Joëls M, Karst H. Circadian and ultradian variations in corticosterone level influence functioning of the male mouse basolateral amygdala. Endocrinology. 2019;160:791–802.
Han F, Ding J, Shi Y. Expression of amygdala mineralocorticoid receptor and glucocorticoid receptor in the single-prolonged stress rats. BMC Neurosci. 2014;15:77.
Willner P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress. 2017;6:78–93.
de Boer SF, Buwalda B, Koolhaas JM. Untangling the neurobiology of coping styles in rodents: Towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci Biobehav Rev. 2017;74:401–22.
Gururajan A, van de Wouw M, Boehme M, Becker T, O’Connor R, Bastiaanssen TFS, et al. Resilience to chronic stress is associated with specific neurobiological, neuroendocrine and immune responses. Brain Behav Immun. 2019;80:583–94.
Murra D, Hilde KL, Fitzpatrick A, Maras PM, Watson SJ, Akil H. Characterizing the behavioral and neuroendocrine features of susceptibility and resilience to social stress. Neurobiol Stress. 2022;17:100437.
Milic M, Schmitt U, Lutz B, Müller MB. Individual baseline behavioral traits predict the resilience phenotype after chronic social defeat. Neurobiol Stress. 2021;14:100290.
Huzard D, Mumby DG, Sandi C, Poirier GL, van der Kooij MA. The effects of extrinsic stress on somatic markers and behavior are dependent on animal housing conditions. Physiol Behav. 2015;151:238–45.
Acknowledgements
MJ is supported by the Consortium on Individual Development (CID), which is funded through the Gravitation program of the Dutch Ministry of Education, Culture, and Science and Netherlands Organization for Scientific Research (project #024.001.003).
Author information
Authors and Affiliations
Contributions
ERdK and MJ contributed equally to conceptualizing and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
ERdK owns stock of Corcept Therapeutics. MJ declares no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Kloet, E.R., Joëls, M. The cortisol switch between vulnerability and resilience. Mol Psychiatry (2023). https://doi.org/10.1038/s41380-022-01934-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-022-01934-8