Abstract
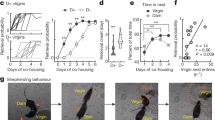
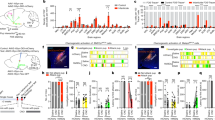
Infant avoidance and aggression are promoted by activation of the Urocortin-3 expressing neurons of the perifornical area of hypothalamus (PeFAUcn3) in male and female mice. PeFAUcn3 neurons have been implicated in stress, and stress is known to reduce maternal behavior. We asked how chronic restraint stress (CRS) affects infant-directed behavior in virgin and lactating females and what role PeFAUcn3 neurons play in this process. Here we show that infant-directed behavior increases activity in the PeFAUcn3 neurons in virgin and lactating females. Chemogenetic inhibition of PeFAUcn3 neurons facilitates pup retrieval in virgin females. CRS reduces pup retrieval in virgin females and increases activity of PeFAUcn3 neurons, while CRS does not affect maternal behavior in lactating females. Inhibition of PeFAUcn3 neurons blocks stress-induced deficits in pup-directed behavior in virgin females. Together, these data illustrate the critical role for PeFAUcn3 neuronal activity in mediating the impact of chronic stress on female infant-directed behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Code availability
Custom Matlab code for analysis of photometry data is available at github.com/babdelme/Autrylab_photometry. MERFISH image analysis scripts are available at github.com/ZhuangLab/MERFISH-analysis.
References
Numan M. The parental brain: mechanisms, development, and evolution. Oxford University Press: New York, 2020, pages cmpp.
Numan M, Insel TR. The neurobiology of parental behavior. Springer-Verlag: New York, 2003, ix, 418 p.pp.
Fischer EK, Roland AB, Moskowitz NA, Tapia EE, Summers K, Coloma LA, et al. The neural basis of tadpole transport in poison frogs. Proc Biol Sci. 2019;286:20191084.
Numan M. Medial preoptic area and maternal behavior in the female rat. J Comp Physiol Psychol. 1974;87:746–59.
Slawski BA, Buntin JD. Preoptic area lesions disrupt prolactin-induced parental feeding behavior in ring doves. Horm Behav. 1995;29:248–66.
Maruska KP, Butler JM, Field KE, Forester C, Augustus A. Neural activation patterns associated with maternal mouthbrooding and energetic state in an African Cichlid Fish. Neuroscience. 2020;446:199–212.
O’Connell LA, Matthews BJ, Hofmann HA. Isotocin regulates paternal care in a monogamous cichlid fish. Horm Behav. 2012;61:725–33.
Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, et al. Functional circuit architecture underlying parental behaviour. Nature. 2018;556:326–31.
Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–30.
Autry AE, Wu Z, Kapoor V, Kohl J, Bambah-Mukku D, Rubinstein ND, et al. Urocortin-3 neurons in the mouse perifornical area promote infant-directed neglect and aggression. Elife. 2021;10:e64680.
Chen PB, Hu RK, Wu YE, Pan L, Huang S, Micevych PE, et al. Sexually dimorphic control of parenting behavior by the medial amygdala. Cell. 2019;176:1206–1221 e1218.
Tsuneoka Y, Tokita K, Yoshihara C, Amano T, Esposito G, Huang AJ, et al. Distinct preoptic-BST nuclei dissociate paternal and infanticidal behavior in mice. EMBO J. 2015;34:2652–70.
Sato K, Hamasaki Y, Fukui K, Ito K, Miyamichi K, Minami M, et al. Amygdalohippocampal area neurons that project to the preoptic area mediate infant-directed attack in male mice. J Neurosci. 2020;40:3981–94.
Paulson JF, Bazemore SD. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta-analysis. JAMA. 2010;303:1961–9.
Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70:490–8.
Zoubovsky SP, Hoseus S, Tumukuntala S, Schulkin JO, Williams MT, Vorhees CV, et al. Chronic psychosocial stress during pregnancy affects maternal behavior and neuroendocrine function and modulates hypothalamic CRH and nuclear steroid receptor expression. Transl Psychiatry. 2020;10:6.
Rosinger ZJ, Mayer HS, Geyfen JI, Orser MK, Stolzenberg DS. Ethologically relevant repeated acute social stress induces maternal neglect in the lactating female mouse. Dev Psychobiol. 2021;63:e22173.
Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;14:677–84.
Wang XD, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, et al. Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. J Neurosci. 2011;31:13625–34.
Singh-Taylor A, Korosi A, Molet J, Gunn BG, Baram TZ. Synaptic rewiring of stress-sensitive neurons by early-life experience: a mechanism for resilience? Neurobiol Stress. 2015;1:109–15.
Rincon-Cortes M, Grace AA. Postpartum scarcity-adversity disrupts maternal behavior and induces a hypodopaminergic state in the rat dam and adult female offspring. Neuropsychopharmacology. 2022;47:488–96.
Kronman H, Torres-Berrio A, Sidoli S, Issler O, Godino A, Ramakrishnan A, et al. Long-term behavioral and cell-type-specific molecular effects of early life stress are mediated by H3K79me2 dynamics in medium spiny neurons. Nat Neurosci. 2021;24:667–76.
Feifel AJ, Shair HN, Schmauss C. Lasting effects of early life stress in mice: interaction of maternal environment and infant genes. Genes Brain Behav. 2017;16:768–80.
Delpech JC, Wei L, Hao J, Yu X, Madore C, Butovsky O, et al. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav Immun. 2016;57:79–93.
Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci Biobehav Rev. 2005;29:843–65.
Brunton PJ, Russell JA, Douglas AJ. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol. 2008;20:764–76.
Walker CD, Tilders FJ, Burlet A. Increased colocalization of corticotropin-releasing factor and arginine vasopressin in paraventricular neurones of the hypothalamus in lactating rats: evidence from immunotargeted lesions and immunohistochemistry. J Neuroendocrinol. 2001;13:74–85.
Medina J, De Guzman RM, Workman JL. Lactation is not required for maintaining maternal care and active coping responses in chronically stressed postpartum rats: Interactions between nursing demand and chronic variable stress. Horm Behav. 2021;136:105035.
Miller SM, Piasecki CC, Lonstein JS. Use of the light-dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharm Biochem Behav. 2011;100:130–7.
Murgatroyd CA, Pena CJ, Podda G, Nestler EJ, Nephew BC. Early life social stress induced changes in depression and anxiety associated neural pathways which are correlated with impaired maternal care. Neuropeptides. 2015;52:103–11.
Murgatroyd CA, Nephew BC. Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology. 2013;38:219–28.
Carini LM, Murgatroyd CA, Nephew BC. Using chronic social stress to model postpartum depression in lactating rodents. J Vis Exp. 2013; e50324.
Neufeld-Cohen A, Kelly PA, Paul ED, Carter RN, Skinner E, Olverman HJ, et al. Chronic activation of corticotropin-releasing factor type 2 receptors reveals a key role for 5-HT1A receptor responsiveness in mediating behavioral and serotonergic responses to stressful challenge. Biol Psychiatry. 2012;72:437–47.
Deussing JM, Breu J, Kuhne C, Kallnik M, Bunck M, Glasl L, et al. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J Neurosci. 2010;30:9103–16.
Fischl MJ, Ueberfuhr MA, Drexl M, Pagella S, Sinclair JL, Alexandrova O, et al. Urocortin 3 signalling in the auditory brainstem aids recovery of hearing after reversible noise-induced threshold shift. J Physiol. 2019;597:4341–55.
Shemesh Y, Forkosh O, Mahn M, Anpilov S, Sztainberg Y, Manashirov S, et al. Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics. Nat Neurosci. 2016;19:1489–96.
Kuperman Y, Issler O, Regev L, Musseri I, Navon I, Neufeld-Cohen A, et al. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc Natl Acad Sci USA. 2010;107:8393–8.
Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology. 2006;147:4578–88.
Venihaki M, Sakihara S, Subramanian S, Dikkes P, Weninger SC, Liapakis G, et al. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviours. J Neuroendocrinol. 2004;16:411–22.
Autry AE, Adachi M, Cheng P, Monteggia LM. Gender-specific impact of brain-derived neurotrophic factor signaling on stress-induced depression-like behavior. Biol Psychiatry. 2009;66:84–90.
Murgatroyd CA, Hicks-Nelson A, Fink A, Beamer G, Gurel K, Elnady F, et al. Effects of Chronic Social Stress and Maternal Intranasal Oxytocin and Vasopressin on Offspring Interferon-gamma and Behavior. Front Endocrinol (Lausanne). 2016;7:155.
Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science. 2018;362:eaau5324.
Chen KH, Boettiger AN, Moffitt JR, Wang S, Zhuang X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348:aaa6090.
Ostermeyer MC, Elwood RW. Pup recognition in Mus musculus: parental discrimination between their own and alien young. Dev Psychobiol. 1983;16:75–82.
Mogi K, Takakuda A, Tsukamoto C, Ooyama R, Okabe S, Koshida N, et al. Mutual mother-infant recognition in mice: The role of pup ultrasonic vocalizations. Behav Brain Res. 2017;325:138–46.
Wittmann G, Fuzesi T, Liposits Z, Lechan RM, Fekete C. Distribution and axonal projections of neurons coexpressing thyrotropin-releasing hormone and urocortin 3 in the rat brain. J Comp Neurol. 2009;517:825–40.
Chen P, Lin D, Giesler J, Li C. Identification of urocortin 3 afferent projection to the ventromedial nucleus of the hypothalamus in rat brain. J Comp Neurol. 2011;519:2023–42.
Carcea I, Caraballo NL, Marlin BJ, Ooyama R, Riceberg JS, Mendoza Navarro JM, et al. Oxytocin neurons enable social transmission of maternal behaviour. Nature. 2021;596:553–7.
Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504.
Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci Biobehav Rev. 2000;24:669–86.
Kuroda KO, Tachikawa K, Yoshida S, Tsuneoka Y, Numan M. Neuromolecular basis of parental behavior in laboratory mice and rats: with special emphasis on technical issues of using mouse genetics. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1205–31.
Herman JP, Tasker JG. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol (Lausanne). 2016;7:137.
Klampfl SM, Bosch OJ. Mom doesn’t care: When increased brain CRF system activity leads to maternal neglect in rodents. Front Neuroendocrinol. 2019;53:100735.
Radley JJ, Sawchenko PE. Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic-pituitary-adrenal axis adaptations to repeated stress. J Comp Neurol. 2015;523:2769–87.
Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–81.
Matovic S, Ichiyama A, Igarashi H, Salter EW, Sunstrum JK, Wang XF, et al. Neuronal hypertrophy dampens neuronal intrinsic excitability and stress responsiveness during chronic stress. J Physiol. 2020;598:2757–73.
Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–29.
Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–13.
Richter K, Wolf G, Engelmann M. Social recognition memory requires two stages of protein synthesis in mice. Learn Mem. 2005;12:407–13.
Chapillon P, Patin V, Roy V, Vincent A, Caston J. Effects of pre- and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: a review. Dev Psychobiol. 2002;41:373–87.
Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–7.
Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–51.
Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann NY Acad Sci. 1997;807:101–25.
Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav. 1980;25:731–43.
Fang YY, Yamaguchi T, Song SC, Tritsch NX, Lin D. A hypothalamic midbrain pathway essential for driving maternal behaviors. Neuron. 2018;98:192–207 e110.
Martin-Sanchez A, Valera-Marin G, Hernandez-Martinez A, Lanuza E, Martinez-Garcia F, Agustin-Pavon C. Wired for motherhood: induction of maternal care but not maternal aggression in virgin female CD1 mice. Front Behav Neurosci. 2015;9:197.
Chalfin L, Dayan M, Levy DR, Austad SN, Miller RA, Iraqi FA, et al. Mapping ecologically relevant social behaviours by gene knockout in wild mice. Nat Commun. 2014;5:4569.
McCarthy MM, Bare JE, vom Saal FS. Infanticide and parental behavior in wild female house mice: effects of ovariectomy, adrenalectomy and administration of oxytocin and prostaglandin F2 alpha. Physiol Behav. 1986;36:17–23.
Konig B. Fitness Effects of Communal Rearing in-House Mice - the Role of Relatedness Versus Familiarity. Anim Behav. 1994;48:1449–57.
Ferrari M, Lindholm AK, Konig B. A reduced propensity to cooperate under enhanced exploitation risk in a social mammal. Proc Biol Sci. 2016;283:20160068.
Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D. Hypothalamic control of male aggression-seeking behavior. Nat Neurosci. 2016;19:596–604.
Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–92.
Gray P, Cooney J. Stress-induced responses and open-field behavior in estrous and nonestrous mice. Physiol Behav. 1982;29:287–92.
Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6:192–200.
Lovick TA, Zangrossi H Jr. Effect of estrous cycle on behavior of females in rodent tests of anxiety. Front Psychiatry. 2021;12:711065.
Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–81.
Ribeiro AC, Musatov S, Shteyler A, Simanduyev S, Arrieta-Cruz I, Ogawa S, et al. siRNA silencing of estrogen receptor-alpha expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc Natl Acad Sci USA. 2012;109:16324–9.
Mann MA, Kinsley C, Broida J, Svare B. Infanticide exhibited by female mice: genetic, developmental and hormonal influences. Physiol Behav. 1983;30:697–702.
Gandelman R. Maternal behavior in the mouse: effect of estrogen and progesterone. Physiol Behav. 1973;10:153–5.
Murakami G. Distinct effects of estrogen on mouse maternal behavior: the contribution of estrogen synthesis in the brain. PLoS One. 2016;11:e0150728.
Ehret G, Schmid C. Reproductive cycle-dependent plasticity of perception of acoustic meaning in mice. Physiol Behav. 2009;96:428–33.
Ehret G, Bernecker C. Low-frequency sound communication by mouse pups (mus-musculus) - wriggling calls release maternal-behavior. Anim Behav. 1986;34:821–30.
Catanese MC, Vandenberg LN. Developmental estrogen exposures and disruptions to maternal behavior and brain: Effects of ethinyl estradiol, a common positive control. Horm Behav. 2018;101:113–24.
Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharm Toxicol. 2007;47:657–80.
Croy A. The guide to investigation of mouse pregnancy. Academic Press: London; Waltham, Massachusetts; San Diego, California, 2014.
Hedrich HJ, ScienceDirect. The laboratory mouse. 2nd edn. Academic Press Elsevier: London Amsterdam, 2012.
Hahn ME, Lavooy MJ. A review of the methods of studies on infant ultrasound production and maternal retrieval in small rodents. Behav Genet. 2005;35:31–52.
Allin JT, Banks EM. Functional aspects of ultrasound production by infant albino rats (Rattus norvegicus). Anim Behav. 1972;20:175–85.
Verhaeghe A, Noirot E. Ultrasound by mouse pups from deaf and hearing strains. Dev Psychobiol. 1978;11:117–24.
Barron S, Segar TM, Yahr JS, Baseheart BJ, Willford JA. The effects of neonatal ethanol and/or cocaine exposure on isolation-induced ultrasonic vocalizations. Pharm Biochem Behav. 2000;67:1–9.
Brunelli SA, Vinocur DD, Soo-Hoo D, Hofer MA. Five generations of selective breeding for ultrasonic vocalization (USV) responses in N:NIH strain rats. Dev Psychobiol. 1997;31:255–65.
Branchi I, Santucci D, Vitale A, Alleva E. Ultrasonic vocalizations by infant laboratory mice: a preliminary spectrographic characterization under different conditions. Dev Psychobiol. 1998;33:249–56.
Hahn ME, Schanz N. The effects of cold, rotation, and genotype on the production of ultrasonic calls in infant mice. Behav Genet. 2002;32:267–73.
Brown RE, Mathieson WB, Stapleton J, Neumann PE. Maternal behavior in female C57BL/6J and DBA/2J inbred mice. Physiol Behav. 1999;67:599–605.
Hennessy MB, Li J, Lowe EL, Levine S. Maternal behavior, pup vocalizations, and pup temperature changes following handling in mice of 2 inbred strains. Dev Psychobiol. 1980;13:573–84.
Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–14.
Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, et al. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl Psychiatry. 2016;6:e702.
Bendesky A, Kwon YM, Lassance JM, Lewarch CL, Yao S, Peterson BK, et al. The genetic basis of parental care evolution in monogamous mice. Nature. 2017;544:434–9.
Deviterne D, Desor D, Krafft B. Maternal behavior variations and adaptations, and pup development within litters of various sizes in Wistar rat. Dev Psychobiol. 1990;23:349–60.
Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 2000;108:215–31.
Perkinson MR, Kirchner MK, Zhang M, Augustine RA, Stern JE, Brown CH. alpha-Melanocyte-stimulating hormone inhibition of oxytocin neurons switches to excitation in late pregnancy and lactation. Physiol Rep. 2022;10:e15226.
Rickenbacher E, Perry RE, Sullivan RM, Moita MA. Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother-pup interactions. Elife. 2017;6:e24080.
Zeng H, Sanes JR. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci. 2017;18:530–46.
Bonaz B, Rivest S. Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. Am J Physiol. 1998;275:R1438–1449.
Johnstone HA, Wigger A, Douglas AJ, Neumann ID, Landgraf R, Seckl JR, et al. Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: changes in feedforward and feedback mechanisms. J Neuroendocrinol. 2000;12:811–22.
Douglas AJ, Brunton PJ, Bosch OJ, Russell JA, Neumann ID. Neuroendocrine responses to stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinology. 2003;144:5268–76.
Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–11.
Johnson SB, Emmons EB, Lingg RT, Anderson RM, Romig-Martin SA, LaLumiere RT, et al. Prefrontal-bed nucleus circuit modulation of a passive coping response set. J Neurosci. 2019;39:1405–19.
Radley J, Morilak D, Viau V, Campeau S. Chronic stress and brain plasticity: Mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci Biobehav Rev. 2015;58:79–91.
Acknowledgements
AEA was supported by a NARSAD Young Investigator Award and a Pathway to Independence Award (NIH R00HD085188). BA was supported by a diversity supplement to AEA’s NIH award (R00HD085188-S1) and a Tishman Scholarship. DB-M was supported by a Pathway to Independence Award (NIH R00HD092542). We thank Catherine Dulac for intellectual input and project support at Harvard University. We thank Stacey Sullivan for assistance with the transfer of data and mice from Harvard University to Albert Einstein College of Medicine. We thank Krysten Garcia for histology assistance. We thank Giovanni Podda for guidance on the photometry analysis. We thank Kostantin Dobrenis, Vladimir Mudragel, and Mariah Marrero for guidance on Axioscan usage. We thank Kevin Fisher for assistance with image export and analysis. We thank all the members of the Autry lab for input on manuscript preparation.
Author information
Authors and Affiliations
Contributions
BA and AEA designed and performed experiments, analyzed, and plotted data, and interpreted data and wrote the paper. RA supported animal experiments and analyzed data. DB-M performed and analyzed the single cell sequencing and MERFISH experiments. IC analyzed and plotted data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelmesih, B., Anderson, R., Bambah-Mukku, D. et al. Urocortin-3 neurons in the perifornical area are critical mediators of chronic stress on female infant-directed behavior. Mol Psychiatry 28, 483–496 (2023). https://doi.org/10.1038/s41380-022-01902-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01902-2