Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder whose pathophysiological mechanisms are still unclear. Hypotheses suggest a role for glutamate dysfunctions in ASD development, but clinical studies investigating brain and peripheral glutamate levels showed heterogenous results leading to hypo- and hyper-glutamatergic hypotheses of ASD. Recently, studies proposed the implication of elevated mGluR5 densities in brain areas in the pathophysiology of ASD. Thus, our objective was to characterize glutamate dysfunctions in adult subjects with ASD by quantifying (1) glutamate levels in the cingulate cortex and periphery using proton magnetic resonance spectroscopy and metabolomics, and (2) mGluR5 brain density in this population and in a validated animal model of ASD (prenatal exposure to valproate) at developmental stages corresponding to childhood and adolescence in humans using positron emission tomography. No modifications in cingulate Glu levels were observed between individuals with ASD and controls further supporting the difficulty to evaluate modifications in excitatory transmission using spectroscopy in this population, and the complexity of its glutamate-related changes. Our imaging results showed an overall increased density in mGluR5 in adults with ASD, that was only observed mostly subcortically in adolescent male rats prenatally exposed to valproic acid, and not detected in the stage corresponding to childhood in the same animals. This suggest that clinical changes in mGluR5 density could reflect the adaptation of the glutamatergic dysfunctions occurring earlier rather than being key to the pathophysiology of ASD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. 2018;392:508–20.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
Chiarotti F, Venerosi A. Epidemiology of autism spectrum disorders: a review of worldwide prevalence estimates since 2014. Brain Sci. 2020;10:274.
Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev. 2012;36:2044–55.
Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems: model of autism. Genes Brain Behav. 2003;2:255–67.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8.
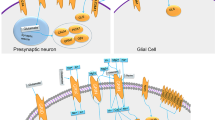
Rojas DC. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J Neural Transm. 2014;121:891–905.
Vyas Y, Cheyne JE, Lee K, Jung Y, Cheung PY, Montgomery JM. Shankopathies in the developing brain in autism spectrum disorders. Front Neurosci. 2021;15:775431.
Eltokhi A, Santuy A, Merchan-Perez A, Sprengel R. Glutamatergic dysfunction and synaptic ultrastructural alterations in schizophrenia and autism spectrum disorder: evidence from human and rodent studies. IJMS. 2020;22:59.
Carlsson ML. Hypothesis: is infantile autism a hypoglutamatergic disorder? Relevance of glutamate—serotonin interactions for pharmacotherapy. J Neural Transm. 1998;105:525.
Fatemi SH. The hyperglutamatergic hypothesis of autism. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:911.
Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57.
Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504.
Lee M, Martin GE, Berry-Kravis E, Losh M. A developmental, longitudinal investigation of autism phenotypic profiles in fragile X syndrome. J Neurodev Disord. 2016;8:47.
Berry-Kravis E, Des Portes V, Hagerman R, Jacquemont S, Charles P, Visootsak J, et al. Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Sci Transl Med. 2016;8:1–11.
Youssef EA, Berry-Kravis E, Czech C, Hagerman RJ, Hessl D, Wong CY, et al. Effect of the mGluR5-NAM Basimglurant on behavior in adolescents and adults with fragile x syndrome in a randomized, double-blind, placebo-controlled trial: FragXis phase 2 results. Neuropsychopharmacology. 2018;43:503–12.
Levenga J, Hayashi S, de Vrij FMS, Koekkoek SK, van der Linde HC, Nieuwenhuizen I, et al. AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome. Neurobiol Dis. 2011;42:311–7.
Hagerman R, Jacquemont S, Berry-Kravis E, Des Portes V, Stanfield A, Koumaras B, et al. Mavoglurant in fragile x syndrome: results of two open-label, extension trials in adults and adolescents. Sci Rep. 2018;8:16970.
Fatemi SH, Folsom TD, Kneeland RE, Liesch SB. Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both fragile x mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec. 2011;294:1635–45.
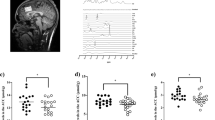
Fatemi SH, Wong DF, Brašić JR, Kuwabara H, Mathur A, Folsom TD, et al. Metabotropic glutamate receptor 5 tracer [18F]-FPEB displays increased binding potential in postcentral gyrus and cerebellum of male individuals with autism: a pilot PET study. Cerebellum Ataxias. 2018;5:3.
Brašić JR, Nandi A, Russell DS, Jennings D, Barret O, Martin SD, et al. Cerebral expression of metabotropic glutamate receptor subtype 5 in idiopathic autism spectrum disorder and fragile x syndrome: a pilot study. IJMS. 2021;22:2863.
Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16.
Hergueta T, Lecrubier Y, Sheehan D, Weiller E. Mini international neuropsychiatric interview French current DSM-IV. 2015. https://doi.org/10.13140/RG.2.1.2792.9440.
First MB. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J Nerv Ment Dis. 2013;201:727–9.
Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85.
Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule–generic: a standard measure of social and communication deficits associated with the spectrum of autism. 2000;30:205–23.
Wechsler D. Echelle d’intelligence de Wechsler pour adultes (WAIS-III). 3ème éd. Paris: ECPA; 2000.
Wechsler D. Echelle d’intelligence de Wechsler pour adultes (WAIS-IV). 4ème éd. Paris: ECPA; 2011.
Schopler E, Lansing MD, Reichler RJ, Marcus LM. PEP-3. Profil psycho-educatif. Evaluation fonctionnelle pour enfants autistes. de BoeckBruxelles, Belgique; 2020.
Barfthelemy C, Roux S, Adrien JL, Hameuiy L, Gtieriis P, Garreau B, et al. Validation of the revised behavior summarized evaluation scale. 1997;27:139–53.
Bourreau Y, Roux S, Gomot M, Bonnet-Brilhault F, Barthélémy C. Validation of the repetitive and restricted behaviour scale in autism spectrum disorders. Eur Child Adolesc Psychiatry. 2009;18:675–82.
Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17.
Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–75.
Dupont A-C, Serrière S, Barantin L, Vercouillie J, Tauber C, Gissot V, et al. Study of influence of the glutamatergic concentration of [18F]FPEB binding to metabotropic glutamate receptor subtype 5 with N-acetylcysteine challenge in rats and SRM/PET study in human healthy volunteers. Transl Psychiatry. 2021;11:66.
Apps MAJ, Lockwood PL, Balsters JH. The role of the midcingulate cortex in monitoring others’ decisions. Front Neurosci. 2013;7:1–7.
Apps MAJ, Rushworth MFS, Chang SWC. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron. 2016;90:692–707.
Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32.
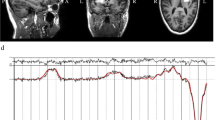
Wilson M, Andronesi O, Barker PB, Bartha R, Bizzi A, Bolan PJ, et al. Methodological consensus on clinical proton MRS of the brain: review and recommendations. Magn Reson Med. 2019;82:527–50.
Oeltzschner G, Zöllner HJ, Hui SCN, Mikkelsen M, Saleh MG, Tapper S, et al. Osprey: open-source processing, reconstruction & estimation of magnetic resonance spectroscopy data. J Neurosci Methods. 2020;343:108827.
Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55:1219–26.
Harris AD, Puts NAJ, Edden RAE. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations: tissue correction for GABA-Edited MRS. J Magn Reson Imaging. 2015;42:1431–40.
Gussew A, Erdtel M, Hiepe P, Rzanny R, Reichenbach JR. Absolute quantitation of brain metabolites with respect to heterogeneous tissue compositions in 1H-MR spectroscopic volumes. Magn Reson Mater Phys. 2012;25:321–33.
Truong V, Duncan NW. Suggestions for improving the visualization of magnetic resonance spectroscopy voxels and spectra. R Soc Open Sci. 2020;7:200600.
Park E, Sullivan JM, Planeta B, Gallezot J-D, Lim K, Lin S-F, et al. Test–retest reproducibility of the metabotropic glutamate receptor 5 ligand [18F]FPEB with bolus plus constant infusion in humans. Eur J Nucl Med Mol Imaging. 2015;42:1530–41.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol). 1995;57:289–300.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
Sawilowsky SS. New effect size rules of thumb. J Mod Appl Stat Meth. 2009;8:597–9.
Blasco H, Bessy C, Plantier L, Lefevre A, Piver E, Bernard L, et al. The specific metabolome profiling of patients infected by SARS-COV-2 supports the key role of tryptophan-nicotinamide pathway and cytosine metabolism. Sci Rep. 2020;10:16824.
Nadler JJ, Moy SS, Dold G, Simmons N, Perez A, Young NB, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–14.
Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–8.
Kammermeier PJ. Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. J Neurosci. 2008;28:8560–7.
Verpelli C, Dvoretskova E, Vicidomini C, Rossi F, Chiappalone M, Schoen M, et al. Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J Biol Chem. 2011;286:34839–50.
Ford TC, Crewther DP. A comprehensive review of the 1H-MRS metabolite spectrum in autism spectrum disorder. Front Mol Neurosci. 2016;9:1–27.
Bernardi S, Anagnostou E, Shen J, Kolevzon A, Buxbaum JD, Hollander E, et al. In vivo 1H-magnetic resonance spectroscopy study of the attentional networks in autism. Brain Res. 2011;1380:198–205.
Bejjani A, O’Neill J, Kim JA, Frew AJ, Yee VW, Ly R, et al. Elevated glutamatergic compounds in pregenual anterior cingulate in pediatric autism spectrum disorder demonstrated by 1H MRS and 1H MRSI. PLoS ONE. 2012;7:e38786.
Joshi G, Biederman J, Wozniak J, Goldin RL, Crowley D, Furtak S, et al. Magnetic resonance spectroscopy study of the glutamatergic system in adolescent males with high-functioning autistic disorder: a pilot study at 4T. Eur Arch Psychiatry Clin Neurosci. 2013;263:379–84.
Ito H, Mori K, Harada M, Hisaoka S, Toda Y, Mori T, et al. A proton magnetic resonance spectroscopic study in autism spectrum disorder using a 3-Tesla clinical magnetic resonance imaging (MRI) system: the anterior cingulate cortex and the left cerebellum. J Child Neurol. 2017;32:731–9.
Naaijen J, Zwiers MP, Forde NJ, Williams SC, Durston S, Brandeis D, et al. Striatal structure and its association with N-Acetylaspartate and glutamate in autism spectrum disorder and obsessive compulsive disorder. Eur Neuropsychopharmacol. 2018;28:118–29.
Siegel-Ramsay JE, Romaniuk L, Whalley HC, Roberts N, Branigan H, Stanfield AC, et al. Glutamate and functional connectivity—support for the excitatory-inhibitory imbalance hypothesis in autism spectrum disorders. Psychiatry Res: Neuroimaging. 2021;313:111302.
Tebartz van Elst L, Maier S, Fangmeier T, Endres D, Mueller GT, Nickel K, et al. Disturbed cingulate glutamate metabolism in adults with high-functioning autism spectrum disorder: evidence in support of the excitatory/inhibitory imbalance hypothesis. Mol Psychiatry. 2014;19:1314–25.
Jiménez-Espinoza C, Marcano Serrano F, González-Mora JL. N-acetylaspartyl-glutamate metabolism in the cingulated cortices as a biomarker of the etiology in ASD: a 1H-MRS model. Molecules. 2021;26:675.
Libero LE, Reid MA, White DM, Salibi N, Lahti AC, Kana RK. Biochemistry of the cingulate cortex in autism: an MR spectroscopy study: biochemistry of the cingulate cortex in autism. Autism Res. 2016;9:643–57.
Shinohe A, Hashimoto K, Nakamura K, Tsujii M, Iwata Y, Tsuchiya KJ, et al. Increased serum levels of glutamate in adult patients with autism. Prog Neuro-Psychopharmacol Biol Psychiatry. 2006;30:1472–7.
Ghanizadeh A. Increased glutamate and homocysteine and decreased glutamine levels in autism: a review and strategies for future studies of amino acids in autism. Dis Markers. 2013;35:281–6.
Zheng H-F, Wang W-Q, Li X-M, Rauw G, Baker GB. Body fluid levels of neuroactive amino acids in autism spectrum disorders: a review of the literature. Amino Acids. 2017;49:57–65.
Lussu M, Noto A, Masili A, Rinaldi AC, Dessì A, De Angelis M, et al. The urinary 1 H-NMR metabolomics profile of an italian autistic children population and their unaffected siblings: metabolomics profile of autistic children. Autism Res. 2017;10:1058–66.
Zaki MM, Abdel-Al H, Al-Sawi M. Assessment of plasma amino acid profile in autism using cation-exchange chromatography with postcolumn derivatization by ninhydrin. Turk J Med Sci. 2017;47:260–67.
Al-Otaish H, Al-Ayadhi L, Bjørklund G, Chirumbolo S, Urbina MA, El-Ansary A. Relationship between absolute and relative ratios of glutamate, glutamine and GABA and severity of autism spectrum disorder. Metab Brain Dis. 2018;33:843–54.
Bitar T, Mavel S, Emond P, Nadal-Desbarats L, Lefèvre A, Mattar H, et al. Identification of metabolic pathway disturbances using multimodal metabolomics in autistic disorders in a Middle Eastern population. J Pharm Biomed Anal. 2018;152:57–65.
Khalifa D, Shahin O, Salem D, Raafat O. Serum glutamate was elevated in children aged 3-10 years with autism spectrum disorders when they were compared with controls. Acta Paediatr. 2019;108:295–9.
Rangel-Huerta OD, Gomez-Fernández A, de la Torre-Aguilar MJ, Gil A, Perez-Navero JL, Flores-Rojas K, et al. Metabolic profiling in children with autism spectrum disorder with and without mental regression: preliminary results from a cross-sectional case–control study. Metabolomics. 2019;15:99.
Gevi F, Belardo A, Zolla L. A metabolomics approach to investigate urine levels of neurotransmitters and related metabolites in autistic children. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165859.
Yu X, Qian-Qian L, Cong Y, Xiao-Bing Z, Hong-Zhu D. Reduction of essential amino acid levels and sex-specific alterations in serum amino acid concentration profiles in children with autism spectrum disorder. Psychiatry Res. 2021;297:113675.
Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M. Individual variability in human blood metabolites identifies age-related differences. Proc Natl Acad Sci USA. 2016;113:4252–9.
Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr. 2009;90:867S–874S.
Gade S, Hjørnevik T, Park JH, Shen B, Gu M, Tseng J, et al. The first awake simultaneous PET-MR study of an adult with fragile X syndrome: a case report. In Review; 2021.
Tartaglione AM, Schiavi S, Calamandrei G, Trezza V. Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder. Neuropharmacology. 2019;159:107477.
Acknowledgements
The clinical study was supported by the French National Agency for Research (“Investissements d’Avenir” no. ANR-11-LABX-0018-01), IRON. This study was funded in part by the “Région centre Val de Loire APR-IA 2014-850”. We thank the staffs at the CERRP, iBrain, CIC 1415, PST-ASB core facility and the staff of the child psychiatry unit and the nuclear medicine department of the university hospital of Tours. We also thank the PRIMEX core facility from the Angers University for the MR-experiments to build the MRI template for male rats at early-adolescence. We thank Jacques Dupont for its technical assistance on preclinical behavioral experiments. We extend our deep gratitude to the participants of this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: FBB, FL, LG, MJSR, PE, and SC; Data acquisition: AL, CB, CR, EHD, FBB, JV, LB, LG, MJSR, SC, SR, and VG; Data analysis: ACD, CT, LB, LG, LN, FB, FBB, MG, MJSR, NA, PE, and SM; Manuscript writing: FB, FBB, LG, MJSR, NA, and PE.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Galineau, L., Arlicot, N., Dupont, AC. et al. Glutamatergic synapse in autism: a complex story for a complex disorder. Mol Psychiatry 28, 801–809 (2023). https://doi.org/10.1038/s41380-022-01860-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01860-9