Abstract
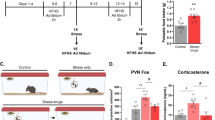
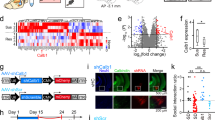
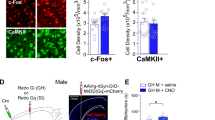
Exposure to trauma is a risk factor for the development of a number of mood disorders, and may enhance vulnerability to future adverse life events. Recent data demonstrate that ventral tegmental area (VTA) neurons expressing the vesicular glutamate transporter 2 (VGluT2) signal and causally contribute to behaviors that involve aversive or threatening stimuli. However, it is unknown whether VTA VGluT2 neurons regulate transsituational outcomes of stress and whether these neurons are sensitive to stressor controllability. This work adapted an operant mouse paradigm to examine the impact of stressor controllability on VTA VGluT2 neuron function as well as the role of VTA VGluT2 neurons in mediating transsituational stressor outcomes. Uncontrollable (inescapable) stress, but not physically identical controllable (escapable) stress, produced social avoidance and exaggerated fear in male mice. Uncontrollable stress in females led to exploratory avoidance of a novel brightly lit environment. Both controllable and uncontrollable stressors increased VTA VGluT2 neuronal activity, and chemogenetic silencing of VTA VGluT2 neurons prevented the behavioral sequelae of uncontrollable stress in male and female mice. Further, we show that stress activates multiple genetically-distinct subtypes of VTA VGluT2 neurons, especially those that are VGluT2+VGaT+, as well as lateral habenula neurons receiving synaptic input from VTA VGluT2 neurons. Our results provide causal evidence that mice can be used for identifying stressor controllability circuitry and that VTA VGluT2 neurons contribute to transsituational stressor outcomes, such as social avoidance, exaggerated fear, or anxiety-like behavior that are observed within trauma-related disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lissek S, van Meurs B. Learning models of PTSD: Theoretical accounts and psychobiological evidence. Int J Psychophysiol: Off J Int Organ Psychophysiol. 2015;98:594–605.
Lissek S, Grillon C. Learning Models of PTSD. The Oxford Handbook of Traumatic Stress Disorders, 1st edn 2012.
Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–9.
Kline AC, Cooper AA, Rytwinksi NK, Feeny NC. Long-term efficacy of psychotherapy for posttraumatic stress disorder: A meta-analysis of randomized controlled trials. Clin Psychol Rev. 2018;59:30–40.
Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin psychiatry. 2013;74:e541–50.
Morina N, Wicherts JM, Lobbrecht J, Priebe S. Remission from post-traumatic stress disorder in adults: A systematic review and meta-analysis of long term outcome studies. 2014;34:249–55.
Markowitz S, Fanselow M. Exposure Therapy for Post-Traumatic Stress Disorder: Factors of Limited Success and Possible Alternative Treatment. Brain Sci. 2020;10:167.
Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76:351–9.
Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85.
Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282:60–8.
Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, et al. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–83.
Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–7.
Root DH, Mejias-Aponte CA, Qi J, Morales M. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J Neurosci. 2014;34:13906–10.
Barbano MF, Wang HL, Zhang S, Miranda-Barrientos J, Estrin DJ, Figueroa-Gonzalez A, et al. VTA Glutamatergic Neurons Mediate Innate Defensive Behaviors. Neuron. 2020;107:368–82.e368
Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–41.
Landgraf D, Long J, Der-Avakian A, Streets M, Welsh DK. Dissociation of learned helplessness and fear conditioning in mice: a mouse model of depression. PLoS One. 2015;10:e0125892.
Greenwood BN, Strong PV, Fleshner M. Lesions of the basolateral amygdala reverse the long-lasting interference with shuttle box escape produced by uncontrollable stress. Behavioural Brain Res. 2010;211:71–6.
Root DH, Barker DJ, Estrin DJ, Miranda-Barrientos JA, Liu B, Zhang S, et al. Distinct Signaling by Ventral Tegmental Area Glutamate, GABA, and Combinatorial Glutamate-GABA Neurons in Motivated Behavior. Cell Rep. 2020;32:108094.
Root DH, Mejias-Aponte CA, Zhang S, Wang HL, Hoffman AF, Lupica CR, et al. Single rodent mesohabenular axons release glutamate and GABA. Nat Neurosci. 2014;17:1543–51.
Root DH, Zhang S, Barker DJ, Miranda-Barrientos J, Liu B, Wang HL, et al. Selective Brain Distribution and Distinctive Synaptic Architecture of Dual Glutamatergic-GABAergic Neurons. Cell Rep. 2018;23:3465–79.
McGovern DJ, Polter AM, Root DH. Neurochemical Signaling of Reward and Aversion to Ventral Tegmental Area Glutamate Neurons. J Neurosci. 2021;41:5471–86.
Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91.
Kessler RC, Stang P, Wittchen HU, Stein M, Walters EE. Lifetime co-morbidities between social phobia and mood disorders in the US National Comorbidity Survey. Psychol Med. 1999;29:555–67.
Root DH, Estrin DJ, Morales M. Aversion or Salience Signaling by Ventral Tegmental Area Glutamate Neurons. iScience. 2018;2:51–62.
Qi J, Zhang S, Wang HL, Barker DJ, Miranda-Barrientos J, Morales M. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat Neurosci. 2016;19:725–33.
Wang HL, Qi J, Zhang S, Wang H, Morales M. Rewarding Effects of Optical Stimulation of Ventral Tegmental Area Glutamatergic Neurons. J Neurosci. 2015;35:15948–54.
Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–7.
Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–85.
Miranda‐Barrientos J, Chambers I, Mongia S, Liu B, Wang HL, Mateo‐Semidey GE, et al. Ventral tegmental area GABA, glutamate, and glutamate‐GABA neurons are heterogeneous in their electrophysiological and pharmacological properties. Eur J Neurosci. 2021;54:4061–84.
Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60.
An XL, Zou JX, Wu RY, Yang Y, Tai FD, Zeng SY, et al. Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Exp Anim. 2011;60:111–23.
Berger S, Gureczny S, Reisinger SN, Horvath O, Pollak DD. Effect of Chronic Corticosterone Treatment on Depression-Like Behavior and Sociability in Female and Male C57BL/6N Mice. Cells 2019;8:1018.
Bondar NP, Lepeshko AA, Reshetnikov VV. Effects of Early-Life Stress on Social and Anxiety-Like Behaviors in Adult Mice: Sex-Specific Effects. Behav Neurol. 2018;2018:1538931.
Karlsson SA, Haziri K, Hansson E, Kettunen P, Westberg L. Effects of sex and gonadectomy on social investigation and social recognition in mice. BMC Neurosci. 2015;16:83.
Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–81.
Rosso M, Wirz R, Loretan AV, Sutter NA, da Cunha CTP, Jaric I, et al. Reliability of Common Mouse Behavioural Tests of Anxiety: a Systematic Review and Meta-Analysis on the Effects of Anxiolytics. Neurosci Biobehav Rev. 2022:104928.
Mingote S, Chuhma N, Kalmbach A, Thomsen GM, Wang Y, Mihali A et al. Dopamine neuron dependent behaviors mediated by glutamate cotransmission. Elife 2017;6:e27566.
Graybuck LT, Daigle TL, Sedeño-Cortés AE, Walker M, Kalmbach B, Lenz GH. et al. Enhancer viruses for combinatorial cell-subclass-specific labeling. Neuron 2021;109:1449–.e641413.
Li D, Yang H, Xiong F, Xu X, Zeng WB, Zhao F et al. Anterograde Neuronal Circuit Tracers Derived from Herpes Simplex Virus 1: Development, Application, and Perspectives. Int J Mol Sci. 2020;21:5937.
Zeng WB, Jiang HF, Gang YD, Song YG, Shen ZZ, Yang H, et al. Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129. Mol Neurodegener. 2017;12:38.
Garcia-Keller C, Kupchik YM, Gipson CD, Brown RM, Spencer S, Bollati F, et al. Glutamatergic mechanisms of comorbidity between acute stress and cocaine self-administration. Mol Psychiatry. 2016;21:1063–9.
Maier SF. Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol Stress. 2015;1:12–22.
Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–8.
Root DH, Wang HL, Liu B, Barker DJ, Mod L, Szocsics P, et al. Glutamate neurons are intermixed with midbrain dopamine neurons in nonhuman primates and humans. Sci Rep. 2016;6:30615.
Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–18.
Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, Shenasa MA, et al. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun. 2016;7:13697.
Asok A, Kandel ER, Rayman JB. The Neurobiology of Fear Generalization. Front Behav Neurosci. 2018;12:329.
Kimble M, Boxwala M, Bean W, Maletsky K, Halper J, Spollen K, et al. The impact of hypervigilance: evidence for a forward feedback loop. J Anxiety Disord. 2014;28:241–5.
Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4:e11352.
Baratta MV, Leslie NR, Fallon IP, Dolzani SD, Chun LE, Tamalunas AM, et al. Behavioural and neural sequelae of stressor exposure are not modulated by controllability in females. Eur J Neurosci. 2018;47:959–67.
Baratta MV, Gruene TM, Dolzani SD, Chun LE, Maier SF, Shansky RM. Controllable stress elicits circuit-specific patterns of prefrontal plasticity in males, but not females. Brain Struct Funct. 2019;224:1831–43.
McNulty CJ, Fallon IP, Amat J, Sanchez RJ, Leslie NR, Root DH, et al. Elevated prefrontal dopamine interferes with the stress-buffering properties of behavioral control in female rats. Neuropsychopharmacology. 2022; https://doi.org/10.1038/s41386-022-01443-w.
Dolzani SD, Baratta MV, Amat J, Agster KL, Saddoris MP, Watkins LR et al. Activation of a Habenulo-Raphe Circuit Is Critical for the Behavioral and Neurochemical Consequences of Uncontrollable Stress in the Male Rat. eNeuro. 2016;3:0229–16.
Lazaridis I, Tzortzi O, Weglage M, Märtin A, Xuan Y, Parent M, et al. A hypothalamus-habenula circuit controls aversion. Mol Psychiatry. 2019;24:1351–68.
Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 2011;470:535–9.
Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cogn Neurosci Rev. 2003;2:198–221.
Bouarab C, Thompson B, Polter AM. VTA GABA Neurons at the Interface of Stress and Reward. Front Neural Circuits. 2019;13:78.
Lowes DC, Chamberlin LA, Kretsge LN, Holt ES, Abbas AI, Park AJ, et al. Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice. Nat Commun. 2021;12:3539.
van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–94.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013;493:532–6.
Ortiz V, Costa Campos R, Fofo H, Fernandez SP, Barik J. Nicotinic receptors promote susceptibility to social stress in female mice linked with neuroadaptations within VTA dopamine neurons. Neuropsychopharmacology. 2022;47:1587–96.
Qi G, Zhang P, Li T, Li M, Zhang Q, He F, et al. NAc-VTA circuit underlies emotional stress-induced anxiety-like behavior in the three-chamber vicarious social defeat stress mouse model. Nat Commun. 2022;13:577.
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013;493:537–41.
Acknowledgements
This research was supported by the Webb-Waring Biomedical Research Award from the Boettcher Foundation (DHR), R01 DA047443 (DHR), F31 MH125569 (DJM), a 2020 NARSAD Young Investigator grant from the Brain and Behavior Research Foundation (DHR), R01 MH050479 (MVB), R21 MH116353 (MVB), American Australian Association Fellowship (MVB), DK078749 (STH), and The University of Colorado Boulder. Ai193 mice were generously provided by the Allen Institute and generated with funds from 1U19MH114830-01. The imaging work was performed at the BioFrontiers Institute Advanced Light Microscopy Core (RRID: SCR_018302). Laser scanning confocal microscopy was performed on a Nikon A1R microscope supported by NIST-CU Cooperative Agreement award number 70NANB15H226. The PerkinElmer Opera Phenix is supported by NIH grant 1S10OD025072. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Prism and Biorender were used to make figures and schematics.
Author information
Authors and Affiliations
Contributions
DJM, MVB, and DHR conceived this project. DJM, AL, KLE, DTH, EDP, SCG, CJM, MVB, and DHR designed, performed, or analyzed behavioral-related experiments. ARR and STH designed, performed, and analyzed whole-cell recording experiments. TLD and BT contributed critical reagents. DJM, MVB, and DHR wrote the manuscript with the contribution of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McGovern, D.J., Ly, A., Ecton, K.L. et al. Ventral tegmental area glutamate neurons mediate nonassociative consequences of stress. Mol Psychiatry (2022). https://doi.org/10.1038/s41380-022-01858-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-022-01858-3