Abstract
Pitt Hopkins Syndrome (PTHS) is a rare syndromic form of autism spectrum disorder (ASD) caused by autosomal dominant mutations in the Transcription Factor 4 (TCF4) gene. TCF4 is a basic helix-loop-helix transcription factor that is critical for neurodevelopment and brain function through its binding to cis-regulatory elements of target genes. One potential therapeutic strategy for PTHS is to identify dysregulated target genes and normalize their dysfunction. Here, we propose that SCN10A is an important target gene of TCF4 that is an applicable therapeutic approach for PTHS. Scn10a encodes the voltage-gated sodium channel Nav1.8 and is consistently shown to be upregulated in PTHS mouse models. In this perspective, we review prior literature and present novel data that suggests inhibiting Nav1.8 in PTHS mouse models is effective at normalizing neuron function, brain circuit activity and behavioral abnormalities and posit this therapeutic approach as a treatment for PTHS.
Similar content being viewed by others
Introduction
Pitt Hopkins Syndrome (PTHS) is a rare neurodevelopmental disorder resulting from autosomal dominant mutations on chromosome 18 at the TCF4 (also known as ITF2, SEF2, E2-2, not T-cell factor 4 which is encoded by TCF7L2 gene) locus. Disease-causing mutations are primarily de novo with rare instances of parental mosaicism [1, 2] and result in TCF4 haploinsufficiency or dominant negative mechanisms [3,4,5,6,7]. PTHS patients display features of ASD and are more generally characterized by intellectual disability, developmental delay, breathing abnormalities, absent or limited speech, motor delay, seizure, constipation, and facial features including wide mouth and a broad nasal base with high bridge [8,9,10,11]. The exact disease-causing mechanisms downstream of TCF4 remains an open question, and to date, no therapeutic interventions have been tested in a clinical trial. However, several studies using PTHS animal models have identified a variety of phenotypes that provide important biological insights into this disorder. These phenotypes are observed across the lifespan, beginning with alterations in cortical development, cell fate specification, neuron development and eventually lead to altered neuronal excitability, synaptic plasticity, and behavioral deficits in adult mice [12,13,14]. Here, we highlight evidence that suggests mutations in Tcf4 lead to ectopic expression of Scn10a/Nav1.8 which partially underlies neuronal excitability, network synchronicity and behavioral deficits observed in PTHS mouse models. Moreover, we discuss evidence that inhibition of Nav1.8 is effective at acutely rescuing these phenotypes and discuss the potential of Nav1.8 as a therapeutic target for the treatment of PTHS (Fig. 1).
TCF4 is imported into the nucleus and forms homo- or heterodimers with itself or other bHLH-domain containing transcription factors. Dimers containing wild-type TCF4 directly bind the genomic locus of Scn10a and repress Scn10a expression. Dimers containing mutant TCF4 are incapable of binding DNA and result in de-repression of Scn10a expression. Resulting ectopic Scn10a/Nav1.8 expression leads to hyperlocomotion, breathing abnormalities and intrinsic excitability deficits which are all rescued by inhibition of Nav1.8.
Identification of SCN10a/Nav1.8 in PTHS
Scn10a/Nav1.8 was first identified as a downstream dysregulated gene of Tcf4 in a rat model of PTHS [6]. In this model system, shRNA and CRISPR/Cas9 constructs specific to Tcf4 were delivered by in utero electroporation leading to cellular transgenesis of layer 2/3 pyramidal neurons and knockdown of Tcf4. This knockdown resulted in a significant reduction in the intrinsic excitability of transfected neurons. Molecular profiling of transfected neurons via translating ribosome affinity purification (iTRAP) led to the identification of two upregulated ion channel genes, Scn10a and Kcnq1. Rescue experiments with antagonists to these two channels and phenocopy experiments via overexpression of Scn10a in wildtype neurons validated the causal role of Scn10a and Kcnq1 in these intrinsic excitability deficits. Further confirmation of the TCF4-dependent excitability deficits was obtained in two different PTHS mouse models. One contained a puromycin cassette replacing the bHLH domain which led to expression of a truncated TCF4 protein (Tcf4+/tr), and the other mouse model contained a missense mutation in the bHLH domain (R579W) which models the R580W mutation commonly found in patients [6, 8, 15]. The germline mutations in both of these mouse models are predicted to produce dominant-negative TCF4 protein [5, 16]. In the Tcf4+/tr mouse model, it was shown that SCN10a expression was upregulated, and consistent with the rat model, pharmacological blockade of Nav1.8 normalized intrinsic excitability deficits [6]. Regulation of Scn10a by Tcf4 appears to be direct, as TCF4 ChIP-seq analysis in rat neuroprogenitor cell cultures indicated that Tcf4 binds directly to regions of the Scn10a genetic locus and therefore is predicted to act as a repressor of Scn10a gene expression in the central nervous system (CNS) [6]. Together, these initial findings indicated Nav1.8 was dysregulated in PTHS rodent models and that its ectopic expression was a key molecular mechanism underlying TCF4-dependent intrinsic excitability deficits (Fig. 1). Fortunately, the unique properties of Nav1.8 make it a suitable drug target.
SCN10a/Nav1.8 function and pharmacology
SCN10a/Nav1.8 is primarily a peripherally expressed, TTX resistant, voltage-gated sodium channel [17]. However, its expression and function in the central nervous system is reported [6, 18, 19] and SCN10a variants are associated with epileptic disorders [20]. In the peripheral nervous system, Nav1.8 is thought to play an important role in nociception [21,22,23,24] and in dorsal root ganglion cells (DRGs) Nav1.8 is responsible for a substantial proportion of the inward current needed to generate an action potential [25]. In addition, Nav1.8 also appears to regulate the frequency of action potential firing and spike-frequency adaptation due to its unique kinetic properties [26, 27]. Nav1.8 channels display prominent slow inactivation [17] and DRGs show a pronounced adaptation of action potential firing in response to stimulation [27]. Selective inhibitors of Nav1.8 have been developed and have shown promise in rodent pain models as well as in early phase human trials. The selective Nav1.8 inhibitor A-803467 has shown significant effects on the maximal amplitude and kinetic properties of the TTX-resistant sodium current in rats [18]. A-803467, exhibited high affinity and selectivity for blocking human Nav1.8 channels and effectively inhibited spontaneous and evoked DRG neuronal action potentials in an in vivo rat preparation. A-803467 also dose-dependently reduced nociception in neuropathic and inflammatory pain models [22]. However, A-803467 in preclinical models has limited oral bioavailability [22]. PF-04531083 (Nav1.8 IC50 = 700 nM) and PF-06305591 (Nav1.8 IC50 = 15 nM) were developed as potent and highly selective Nav1.8 inhibitors with acceptable oral bioavailability and showed effectiveness in preclinical pain models [28, 29]. PF-04531083 was tested in a clinical trial for treatment of post-surgical dental pain and was found to have no serious adverse side effects [30]. Moreover, PF-04531083 can pass the blood brain barrier (data not shown), and was shown to rescue CNS phenotypes in a PTHS mouse model, whereas PF-06305591 is non brain penetrant (Fig. 1) [19, 28, 29]. More recently, VX-548 an oral selective Nav1.8 inhibitor has shown success in two phase 2 clinical trials for acute pain in patients who had recently undergone abdominoplasty or bunionectomy [31, 32], however the ability of VX-548 to penetrate the blood brain barrier is not reported.
Normalization of breathing and behavioral abnormalities
A common symptom observed in PTHS patients is disordered breathing characterized by hyperventilation and intermittent apnea or breath holding [33, 34]. These breathing abnormalities severely impact the patient’s quality of life and often contribute to aspiration-induced pneumonia, which is the leading cause of death in PTHS [35, 36]. Remarkably, similar breathing abnormalities were observed in a PTHS mouse model [19]. Tcf4+/tr mice display frequent episodes of hyperventilation, reduced sigh activity, increased post-sigh apnea, and fail to increase inspiratory and expiratory output in response to CO2 (Fig. 1). Cleary and colleagues deduced that these breathing abnormalities may result from abnormal function of the retrotrapezoid nucleus (RTN) because similar breathing abnormalities are found in Rett Syndrome and are known to involve chemoreception. In addition, acetazolamide, a carbonic anhydrase inhibitor, used to induce metabolic acidosis and hyperventilation, improved breathing in PTHS patients [37,38,39,40,41,42]. They showed that TCF4 mutation resulted in selective loss of parafacial Phox2b+ neurons, altered connectivity between Phox2b+ neurons and the pre-BotC complex, and suppressed excitability of chemosensitive RTN neurons. All these phenotypes were consistent with previously observed phenotypes in various brain regions of PTHS mouse models [6, 15, 43, 44]. They demonstrated that Scn10a expression is not normally detected in the RTN of Tcf4+/+ mice, however Scn10a expression was observed in Tcf4+/tr mice. Remarkably, pharmacological block of Nav1.8 with i.p. injection of PF-04531083 was effective at rescuing breathing abnormalities in Tcf4+/tr mice. Moreover, they showed that acute Nav1.8 block was also effective at rescuing hyperlocomotion and anxiety (Fig. 1). Importantly, they demonstrated that rescue by PF-04531083 was specific to inhibition of Nav1.8 in the CNS, because i.p. injection of PF-06305591, which does not penetrate the blood brain barrier, was ineffective at normalizing behavior.
Together, Cleary and colleagues provided direct in vivo evidence showing that central inhibition of Nav1.8 was effective at normalizing breathing and behavioral abnormalities in a PTHS mouse model, further supporting the idea of Nav1.8 as a therapeutic target. In another set of studies, Ekins and colleagues performed a high throughput screen to identify FDA approved drugs for inhibition on recombinant Nav1.8 expressed in HEK cells [45]. Their screen identified a number of dihydropyridine calcium channel antagonists that were effective at blocking Nav1.8 channels, with nicardipine being the most potent having a sub micromolar IC50 (0.6 µM). They went on to show that administration of nicardipine improved several behavioral deficits in a PTHS mouse model, including social recognition, nesting, self-grooming, fear conditioning, and hyperlocomotion [45]. However, the exact mechanism of rescue by nicardipine is not entirely clear, as it is likely inhibiting both sodium and calcium channels. Overall, these studies provide evidence that inhibition of Nav1.8 is effective at rescuing breathing and behavioral abnormalities in PTHS mouse models and therefore support therapeutic targeting of Nav1.8.
Normalization of auditory evoked potentials
Event-related potentials (ERPs) are stereotyped patterns of voltage fluctuation measured in response to sensory stimuli, which consist of temporal components that reflect physiological response. Levels of spectral power and phase coherence during ERP components are thought to reflect strength and connectivity in cortical circuits that mediate sensory information processing [46]. Following our previously published methods [47], we recorded auditory ERPs in 12 week old C57Bl6/J male wild-type (Tcf4+/+) and Tcf4+/tr mice approximately 35 min after vehicle treatment (baseline) or acute administration of the Nav1.8 antagonist PF-04531083 (i.p., 10 mg/kg). We used component and time-frequency analysis of the ERP to identify changes in patterns of synchronized oscillatory activity during the ERP in this PTHS mouse model at baseline and following Nav1.8 antagonism. Component analysis of the ERP showed that there is a significant effect of genotype in reducing the N40 amplitude peak (Fig. 2B–D). In addition, the event-related spectral perturbation (ERSP) power showed changes relative to tone onset in Tcf4+/tr mice and alterations in phase locking as measured by intertrial phase coherence (ITC, Fig. 3). Specifically, we observed no difference in ERSP at baseline between genotypes (Fig. 3A and data not shown) but observed significant delay in the latency of low (theta) frequency activity (Fig. 3B, E), and increased level of coherence in the high (gamma) frequency ITC at baseline (Fig. 3B, D). The delayed oscillatory activity and increased gamma synchrony in response to auditory stimuli suggests impairments in the neural correlates of sensory information processing in this PTHS mouse model. The N40 in rodents is thought to be functionally analogous to the P50 in humans. A reduction in P50 amplitude has been strongly associated with schizophrenia and validated in both genetic and pharmacological rodent models of schizophrenia [48,49,50]. Gamma synchrony is often associated with the function of fast-spiking parvalbumin-positive interneurons [51, 52] and therefore the observed alterations in this frequency band could indicate abnormal functioning of these gabaergic interneurons in the PTHS mouse model. Given prior evidence that Nav1.8 is upregulated in this mouse model and that Nav1.8 antagonists were effective at normalizing both intrinsic excitability and behavior, we quantified the acute effect of PF-04531083 on ERPs. PF-04531083 had no effect on N40 peak amplitude in Tcf4+/+ or Tcf4+/tr mice (Fig. 2B–D), suggesting this particular aspect of the AEP is unrelated to Nav1.8 and results from additional mechanisms downstream of TCF4. However, PF-04531083 did significantly reduce gamma ERSP in Tcf4+/tr mice, but not in Tcf4+/+ mice (Fig. 3A, C). In addition, acute Nav1.8 blockade also normalized the latency of theta ITC and gamma ITC (Fig. 3B, D, E). These results suggest acute Nav1.8 antagonism is effective at normalizing abnormal synchronous activity in the PTHS mouse model and provides further support that Nav1.8 may have utility for treating symptoms in PTHS. Moreover, these data represent a potential electrophysiological biomarker that could be utilized for screening Nav1.8 antagonists for therapeutic efficacy. Patterns of oscillatory activity are well-conserved across species, and if similarly altered ERP responses were detected by scalp EEG recordings in PTHS patients, the translational worth of this biomarker would be invaluable.
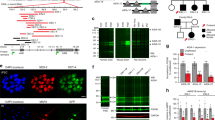
A Example event-related potential (ERP) grand averages from individual temporal components (P20, N40, P80 and P120) where time 0 = auditory stimulus onset. B Grand average ERPs in Tcf4+/tr (n = 10) compared to Tcf4+/+ (n = 12) animals at baseline and (C) following PF-04531083 administration. D Summary component analysis showing significantly reduced amplitudes in N40 peaks in Tcf4+/tr mice compared to Tcf4+/+ animals, which are not altered by PF-04531083 administration (2-way RM ANOVA, *p = 0.0163, main effect of genotype; ns p = 0.1317, main effect of treatment). All descriptive statistics and p values for the data presented in this figure are provided in Supplementary Table 1.
A Heat maps of event-related spectral perturbation (ERSP) in Tcf4+/+ (left, n = 12) and Tcf4+/tr (right, n = 10) animals depicting ERP-related changes due to genotype (vehicle) and rescue with PF-04531083 (SCN10a). B Heat maps of intertrial coherence (ITC) in Tcf4+/+ (left, n = 12) and Tcf4+/tr (right, n = 10) animals depicting ERP-related changes due to genotype (vehicle) and rescue with PF-04531083 (SCN10a). C Reduction of gamma ERSP following SCN10a antagonism in Tcf4+/tr, but not in Tcf4+/+ animals (2-way RM ANOVA, p = 0.0453 interaction of genotype X treatment; Bonferroni post hoc, *p = 0.0269 vehicle-treated Tcf4+/tr versus PF-04531083-treated Tcf4+/tr; ns p > 0.05 vehicle-treated Tcf4+/+ versus PF-04531083-treated Tcf4+/+). D High frequency disturbances in Tcf4+/tr mice are corrected by SCN10a antagonist. There is significantly higher gamma ITC in vehicle-treated Tcf4+/tr compared to Tcf4+/+ vehicle-treated mice in the first 75 ms post-tone. Following SCN10a treatment, there is no effect of genotype (2-way RM ANOVA, p = 0.0027 interaction of genotype X treatment; Bonferroni post hoc, *p = 0.0446 vehicle-treated Tcf4+/+ versus Tcf4+/tr; ns p > 0.05 PF-04531083-treated Tcf4+/+ versus Tcf4+/tr). E Low frequency disturbances in Tcf4+/tr mice are corrected by PF-04531083. Latency to peak theta (3–8 Hz) ITC is significantly increased in vehicle-treated Tcf4+/tr compared to vehicle-treated Tcf4+/+ mice. No significant effect of genotype is detected following treatment with the SCN10a antagonist (2-way RM ANOVA, p = 0.0123 interaction of genotype X treatment; Bonferroni post hoc, *p = 0.0303 vehicle-treated Tcf4+/+ versus Tcf4+/tr; ns p > 0.05 PF-04531083-treated Tcf4+/+ versus Tcf4+/tr).
SCN10a/Nav1.8 and myelination
Another mechanistic link that supports the use of Nav1.8 antagonists for the treatment of PTHS could be through its relation to demyelinating disorders. Transcriptional profiling of several different PTHS mouse models showed that differentially expressed genes were enriched in neurons and oligodendrocytes (OLs), and analysis of OLs and myelination in the Tcf4+/tr mouse showed a significant reduction in OL density, myelination and function [43]. These results suggest that re-myelination could be a potential therapeutic avenue for PTHS but may also provide another link to therapeutic targeting of Scn10a/Nav1.8. Several groups have shown that a variety of diseases associated with demyelination result in maladaptive ectopic expression of Scn10a/Nav1.8. For instance, hereditary demyelinating neuropathy leads to an upregulation of Scn10a/Nav1.8 and abnormal axonal excitability [53], and ectopic Scn10a/Nav1.8 is observed in the cerebellum of the experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis (MS) and in MS patients [54]. Motor deficits are common to both MS and PTHS patients and it has been observed that paroxysmal dysarthria and ataxia in MS patients responds to treatment with sodium channel blocker such as carbamazepine [55, 56]. These results have led to the notion that Nav1.8 antagonists may be a beneficial treatment for demyelinating diseases and neuropathies [24]. It was subsequently shown that an administration of an orally bioavailable Nav1.8 antagonist (PF-01247324) improved cerebellar-dependent motor coordination in a transgenic mouse model overexpressing Scn10a as well as the EAE mouse model of MS [57, 58]. The link between demyelination and Scn10a expression is intriguing, and a similar maladaptive mechanism could be at play in PTHS in response to the TCF4-dependent reduction in myelination. Overall, these results suggest inhibition of Nav1.8 in PTHS patients may provide a dual benefit by normalizing neuronal excitability and improving myelin related excitability deficits.
Conclusion
Currently there are no approved medications for the core symptoms of ASD or even subsets of ASD like PTHS. Here, we discuss the results of a variety of rodent studies on PTHS that all converge on Nav1.8 as being a plausible therapeutic target. Rodent models of PTHS have routinely shown that disruption of Tcf4 function leads to upregulation of Scn10a/Nav1.8 and pharmacological blockade of Nav1.8 is effective at normalizing both physiological and behavioral phenotypes (Fig. 1). Potent and selective Nav1.8 antagonists are developed and their safety in humans is demonstrated in clinical trials [59, 60]. Given all these factors, we recommend testing antagonists of Nav1.8 as a therapeutic approach for PTHS.
References
Steinbusch CVM, van Roozendaal KEP, Tserpelis D, Smeets EEJ, Kranenburg-de Koning TJ, de Waal KH, et al. Somatic mosaicism in a mother of two children with Pitt-Hopkins syndrome. Clin Genet. 2013;83:73–7.
Kousoulidou L, Tanteles G, Moutafi M, Sismani C, Patsalis PC, Anastasiadou V. 263.4 kb deletion within the TCF4 gene consistent with Pitt-Hopkins syndrome, inherited from a mosaic parent with normal phenotype. Eur J Med Genet. 2013;56:314–8.
Zweier C, Sticht H, Bijlsma EK, Clayton-Smith J, Boonen SE, Fryer A, et al. Further delineation of Pitt-Hopkins syndrome: phenotypic and genotypic description of 16 novel patients. J Med Genet. 2008;45:738–44.
Amiel J, Rio M, de Pontual L, Redon R, Malan V, Boddaert N, et al. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am J Hum Genet. 2007;80:988–93.
Sepp M, Pruunsild P, Timmusk T. Pitt-Hopkins syndrome-associated mutations in TCF4 lead to variable impairment of the transcription factor function ranging from hypomorphic to dominant-negative effects. Hum Mol Genet. 2012;21:2873–88.
Rannals MD, Hamersky GR, Page SC, Campbell MN, Briley A, Gallo RA, et al. Psychiatric risk gene transcription factor 4 regulates intrinsic excitability of prefrontal neurons via repression of scn10a and KCNQ1. Neuron 2016;90:43–55.
Brockschmidt A, Filippi A, Charbel Issa P, Nelles M, Urbach H, Eter N, et al. Neurologic and ocular phenotype in Pitt-Hopkins syndrome and a zebrafish model. Hum Genet. 2011;130:645–55.
Whalen S, Héron D, Gaillon T, Moldovan O, Rossi M, Devillard F, et al. Novel comprehensive diagnostic strategy in Pitt-Hopkins syndrome: clinical score and further delineation of the TCF4 mutational spectrum. Hum Mutat. 2012;33:64–72.
Forrest M, Chapman RM, Doyle AM, Tinsley CL, Waite A, Blake DJ. Functional analysis of TCF4 missense mutations that cause Pitt-Hopkins syndrome. Hum Mutat. 2012;33:1676–86.
Sweatt JD. Pitt-Hopkins Syndrome: intellectual disability due to loss of TCF4-regulated gene transcription. Exp Mol Med. 2013;45:e21.
Watkins A, Bissell S, Moss J, Oliver C, Clayton-Smith J, Haye L, et al. Behavioural and psychological characteristics in Pitt-Hopkins syndrome: a comparison with Angelman and Cornelia de Lange syndromes. J Neurodev Disord. 2019;11:24.
Chen H-Y, Bohlen JF, Maher BJ. Molecular and cellular function of transcription factor 4 in Pitt-Hopkins syndrome. Dev Neurosci. 2021;43:159–67.
Rannals MD, Maher BJ. Molecular mechanisms of transcription factor 4 in pitt hopkins syndrome. Curr Genet Med Rep. 2017;5:1–7.
Teixeira JR, Szeto RA, Carvalho VMA, Muotri AR, Papes F. Transcription factor 4 and its association with psychiatric disorders. Transl Psychiatry. 2021;11:19.
Thaxton C, Kloth AD, Clark EP, Moy SS, Chitwood RA, Philpot BD. Common pathophysiology in multiple mouse models of Pitt-Hopkins Syndrome. J Neurosci. 2018;38:918–36.
Rannals MD, Page SC, Campbell MN, Gallo RA, Mayfield B, Maher BJ. Neurodevelopmental models of transcription factor 4 deficiency converge on a common ion channel as a potential therapeutic target for Pitt Hopkins syndrome. Rare Dis. 2016;4:e1220468.
Vijayaragavan K, O’Leary ME, Chahine M. Gating properties of Na(v)1.7 and Na(v)1.8 peripheral nerve sodium channels. J Neurosci. 2001;21:7909–18.
Szulczyk B, Pasierski M, Gawlak M. Prefrontal cortex pyramidal neurons express functional Nav1.8 tetrodotoxin-resistant sodium currents. Clin Exp Pharm Physiol. 2022;49:350–9.
Cleary CM, James S, Maher BJ, Mulkey DK. Disordered breathing in a Pitt-Hopkins syndrome model involves Phox2b-expressing parafacial neurons and aberrant Nav1.8 expression. Nat Commun. 2021;12:5962.
Kambouris M, Thevenon J, Soldatos A, Cox A, Stephen J, Ben-Omran T, et al. Biallelic SCN10A mutations in neuromuscular disease and epileptic encephalopathy. Ann Clin Transl Neurol. 2017;4:26–35.
Gold MS, Weinreich D, Kim C-S, Wang R, Treanor J, Porreca F, et al. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–66.
Jarvis MF, Honore P, Shieh C-C, Chapman M, Joshi S, Zhang X-F, et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA. 2007;104:8520–5.
Joshi SK, Mikusa JP, Hernandez G, Baker S, Shieh C-C, Neelands T, et al. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. Pain 2006;123:75–82.
Han C, Huang J, Waxman SG. Sodium channel Nav1.8: emerging links to human disease. Neurology 2016;86:473–83.
Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–40.
Dib-Hajj SD, Tyrrell L, Cummins TR, Black JA, Wood PM, Waxman SG. Two tetrodotoxin-resistant sodium channels in human dorsal root ganglion neurons. FEBS Lett. 1999;462:117–20.
Blair NT, Bean BP. Role of tetrodotoxin-resistant Na+ current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons. J Neurosci. 2003;23:10338–50.
Bagal SK, Bungay PJ, Denton SM, Gibson KR, Glossop MS, Hay TL, et al. Discovery and optimization of selective nav1.8 modulator series that demonstrate efficacy in preclinical models of pain. ACS Med Chem Lett. 2015;6:650–4.
Brown AD, Bagal SK, Blackwell P, Blakemore DC, Brown B, Bungay PJ, et al. The discovery and optimization of benzimidazoles as selective NaV1.8 blockers for the treatment of pain. Bioorg Med Chem. 2019;27:230–9.
Efficacy Of Pf-04531083 In Treating Post-Surgical Dental Pain - Full Text View - ClinicalTrials.gov [Internet]. [cited 2022 Jun]. Available from: https://www.clinicaltrials.gov/ct2/show/study/NCT01512160?term=04531083&draw=2&rank=6.
Vertex Pharmaceuticals Incorporated. A Study Evaluating Efficacy and Safety of VX-548 for Acute Pain After a Bunionectomy. 2022 [cited 2022 Apr]. Available from: https://clinicaltrials.gov/ct2/show/NCT04977336?term=VX-548&draw=2&rank=1.
Vertex Pharmaceuticals Incorporated. A Study Evaluating Efficacy and Safety of VX-548 for Acute Pain After an Abdominoplasty. 2022 [cited 2022 Apr]. Available from: https://clinicaltrials.gov/ct2/show/NCT05034952?term=VX-548&draw=2&rank=2.
de Winter CF, Baas M, Bijlsma EK, van Heukelingen J, Routledge S, Hennekam RCM. Phenotype and natural history in 101 individuals with Pitt-Hopkins syndrome through an internet questionnaire system. Orphanet J Rare Dis. 2016;11:37.
Goodspeed K, Newsom C, Morris MA, Powell C, Evans P, Golla S. Pitt-Hopkins syndrome: a review of current literature, clinical approach, and 23-patient case series. J Child Neurol. 2018;33:233–44.
Marangi G, Zollino M. Pitt-Hopkins syndrome and differential diagnosis: a molecular and clinical challenge. J Pediatr Genet. 2015;4:168–76.
Hasi M, Soileau B, Sebold C, Hill A, Hale DE, O’Donnell L, et al. The role of the TCF4 gene in the phenotype of individuals with 18q segmental deletions. Hum Genet. 2011;130:777–87.
Willemsen MH, Rensen JHM, van Schrojenstein-Lantman de Valk HMJ, Hamel BCJ, Kleefstra T. Adult Phenotypes in Angelman- and Rett-Like Syndromes. Mol Syndromol. 2012;2:217–34.
Garg SK, Lioy DT, Knopp SJ, Bissonnette JM. Conditional depletion of methyl-CpG-binding protein 2 in astrocytes depresses the hypercapnic ventilatory response in mice. J Appl Physiol. 2015;119:670–6.
Zhang X, Su J, Cui N, Gai H, Wu Z, Jiang C. The disruption of central CO2 chemosensitivity in a mouse model of Rett syndrome. Am J Physiol, Cell Physiol. 2011;301:C729–38.
Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med. 2002;165:1251–60.
Verhulst SL, De Dooy J, Ramet J, Bockaert N, Van Coster R, Ceulemans B, et al. Acetazolamide for severe apnea in Pitt-Hopkins syndrome. Am J Med Genet A 2012;158A:932–4.
Gaffney C, McNally P. Successful use of acetazolamide for central apnea in a child with Pitt-Hopkins syndrome. Am J Med Genet A 2015;167:1423.
Phan BN, Bohlen JF, Davis BA, Ye Z, Chen H-Y, Mayfield B, et al. A myelin-related transcriptomic profile is shared by Pitt-Hopkins syndrome models and human autism spectrum disorder. Nat Neurosci. 2020;23:375–85.
Li H, Zhu Y, Morozov YM, Chen X, Page SC, Rannals MD, et al. Disruption of TCF4 regulatory networks leads to abnormal cortical development and mental disabilities. Mol Psychiatry. 2019;24:1235–46.
Ekins S, Gerlach J, Zorn KM, Antonio BM, Lin Z, Gerlach A. Repurposing approved drugs as inhibitors of kv7.1 and nav1.8 to treat pitt hopkins syndrome. Pharm Res. 2019;36:137.
Featherstone RE, McMullen MF, Ward KR, Bang J, Xiao J, Siegel SJ. EEG biomarkers of target engagement, therapeutic effect, and disease process. Ann N. Y Acad Sci. 2015;1344:12–26.
Hill JL, Hardy NF, Jimenez DV, Maynard KR, Kardian AS, Pollock CJ, et al. Loss of promoter IV-driven BDNF expression impacts oscillatory activity during sleep, sensory information processing and fear regulation. Transl Psychiatry. 2016;6:e873.
Amann LC, Gandal MJ, Halene TB, Ehrlichman RS, White SL, McCarren HS, et al. Mouse behavioral endophenotypes for schizophrenia. Brain Res Bull. 2010;83:147–61.
Smucny J, Stevens KE, Olincy A, Tregellas JR. Translational utility of rodent hippocampal auditory gating in schizophrenia research: a review and evaluation. Transl Psychiatry. 2015;5:e587.
Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700.
Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–40.
Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–13.
Moldovan M, Rosberg MR, Alvarez S, Klein D, Martini R, Krarup C. Aging-associated changes in motor axon voltage-gated Na(+) channel function in mice. Neurobiol Aging. 2016;39:128–39.
Black JA, Dib-Hajj S, Baker D, Newcombe J, Cuzner ML, Waxman SG. Sensory neuron-specific sodium channel SNS is abnormally expressed in the brains of mice with experimental allergic encephalomyelitis and humans with multiple sclerosis. Proc Natl Acad Sci USA. 2000;97:11598–602.
Andermann F, Cosgrove JB, Lloyd-Smith D, Walters AM. Paroxysmal dysarthria and ataxia in multiple sclerosis; a report of 2 unusual cases. Neurology 1959;9:211–5.
Espir ML, Watkins SM, Smith HV. Paroxysmal dysarthria and other transient neurological disturbances in disseminated sclerosis. J Neurol Neurosurg Psychiatr. 1966;29:323–30.
Payne CE, Brown AR, Theile JW, Loucif AJC, Alexandrou AJ, Fuller MD, et al. A novel selective and orally bioavailable Nav 1.8 channel blocker, PF-01247324, attenuates nociception and sensory neuron excitability. Br J Pharm. 2015;172:2654–70.
Shields SD, Butt RP, Dib-Hajj SD, Waxman SG. Oral administration of PF-01247324, a subtype-selective Nav1.8 blocker, reverses cerebellar deficits in a mouse model of multiple sclerosis. PLoS ONE. 2015;10:e0119067.
Hijma HJ, Siebenga PS, de Kam ML, Groeneveld GJ. A phase 1, randomized, double-blind, placebo-controlled, crossover study to evaluate the pharmacodynamic effects of VX-150, a highly selective NaV1.8 inhibitor, in healthy male adults. Pain Med. 2021;22:1814–26.
Hijma HJ, van Brummelen EMJ, Siebenga PS, Groeneveld GJ. A phase I, randomized, double-blind, placebo-controlled, single- and multiple dose escalation study evaluating the safety, pharmacokinetics and pharmacodynamics of VX-128, a highly selective Nav 1.8 inhibitor, in healthy adults. Clin Transl Sci. 2022;15:981–93.
Acknowledgements
We are grateful for the vision and generosity of the Lieber and Maltz families, who made this work possible. This work was supported by the Lieber Institute for Brain Development, the Pitt–Hopkins Research Foundation Awards (to BJM), National Institute of Mental Health (NIMH) grant R56MH104593 (to BJM), NIMH grant R01MH110487 (to BJM). We thank Julia Hill for performing and analyzing experiments.
Author information
Authors and Affiliations
Contributions
KM and BJM designed the experiments. YM performed and analyzed the experiments. KM analyzed the results. DD, SRS and BJM wrote the manuscript. RFK generated the illustration. KM and BJM reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martinowich, K., Das, D., Sripathy, S.R. et al. Evaluation of Nav1.8 as a therapeutic target for Pitt Hopkins Syndrome. Mol Psychiatry 28, 76–82 (2023). https://doi.org/10.1038/s41380-022-01811-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01811-4
This article is cited by
-
Exosome lncRNA IFNG-AS1 derived from mesenchymal stem cells of human adipose ameliorates neurogenesis and ASD-like behavior in BTBR mice
Journal of Nanobiotechnology (2024)
-
Psychiatric risk gene Transcription Factor 4 (TCF4) regulates the density and connectivity of distinct inhibitory interneuron subtypes
Molecular Psychiatry (2023)