Abstract
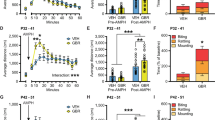
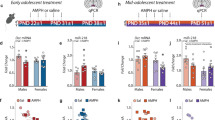
Virtually all neuropsychiatric disorders display sex differences in prevalence, age of onset, and/or clinical symptomology. Although altered dopamine (DA) signaling is a feature of many of these disorders, sex-dependent mechanisms uniquely responsive to DA that drive sex-dependent behaviors remain unelucidated. Previously, we established that anomalous DA efflux (ADE) is a prominent feature of the DA transporter (DAT) variant Val559, a coding substitution identified in two male-biased disorders: attention-deficit/hyperactivity disorder and autism spectrum disorder. In vivo, Val559 ADE induces activation of nigrostriatal D2-type DA autoreceptors (D2ARs) that magnifies inappropriate, nonvesicular DA release by elevating phosphorylation and surface trafficking of ADE-prone DAT proteins. Here we demonstrate that DAT Val559 mice exhibit sex-dependent alterations in psychostimulant responses, social behavior, and cognitive performance. In a search for underlying mechanisms, we discovered that the ability of ADE to elicit D2AR regulation of DAT is both sex and circuit-dependent, with dorsal striatum D2AR/DAT coupling evident only in males, whereas D2AR/DAT coupling in the ventral striatum is exclusive to females. Moreover, systemic administration of the D2R antagonist sulpiride, which precludes ADE-driven DAT trafficking, can normalize DAT Val559 behavioral changes unique to each sex and without effects on the opposite sex or wildtype mice. Our studies support the sex- and circuit dependent capacity of D2ARs to regulate DAT as a critical determinant of the sex-biased effects of perturbed DA signaling in neurobehavioral disorders. Moreover, our work provides a cogent example of how a shared biological insult drives alternative physiological and behavioral trajectories as opposed to resilience.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cox J, Witten IB. Striatal circuits for reward learning and decision-making. Nat Rev Neurosci. 2019;20:482–494.
Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85.
Gogos A, Ney LJ, Seymour N, Van Rheenen TE, Felmingham KL. Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: Are gonadal hormones the link? Br J Pharm. 2019;176:4119–4135.
Mowlem FD, Rosenqvist MA, Martin J, Lichtenstein P, Asherson P, Larsson H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur Child Adolesc Psychiatry. 2019;28:481–489.
Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. 2017;95:136–147.
Gottgens I, van Halteren AD, de Vries NM, Meinders MJ, Ben-Shlomo Y, Bloem BR, et al. The impact of sex and gender on the multidisciplinary management of care for persons with Parkinson’s disease. Front Neurol. 2020;11:576121.
Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612.
Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1998;95:4029–4034.
Benoit-Marand M, Jaber M, Gonon F. Release and elimination of dopamine in vivo in mice lacking the dopamine transporter: functional consequences. Eur J Neurosci. 2000;12:2985–2992.
Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070.
Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017;8:13877.
Dluzen DE, McDermott JL. Sex differences in dopamine- and vesicular monoamine-transporter functions. Ann N. Y Acad Sci. 2008;1139:140–150.
Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–1202.
Foster JD, Yang JW, Moritz AE, Challasivakanaka S, Smith MA, Holy M, et al. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J Biol Chem. 2012;287:29702–29712.
Brundage JN, Mason CP, Wadsworth HA, Finuf CS, Nelson JJ, Ronstrom PJW, et al. Regional and sex differences in spontaneous striatal dopamine transmission. J Neurochem. 2021;160:598–612.
Becker JB, McClellan M, Reed BG. Sociocultural context for sex differences in addiction. Addict Biol. 2016;21:1052–1059.
Bartz D, Chitnis T, Kaiser UB, Rich-Edwards JW, Rexrode KM, Pennell PB, et al. Clinical Advances in Sex- and Gender-Informed Medicine to Improve the Health of All: A Review. JAMA Intern Med. 2020;180:574–583.
Mergy MA, Gowrishankar R, Davis GL, Jessen TN, Wright J, Stanwood GD, et al. Genetic targeting of the amphetamine and methylphenidate-sensitive dopamine transporter: on the path to an animal model of attention-deficit hyperactivity disorder. Neurochem Int. 2014;73:56–70.
Gowrishankar R, Hahn MK, Blakely RD. Good riddance to dopamine: roles for the dopamine transporter in synaptic function and dopamine-associated brain disorders. Neurochem Int. 2014;73:42–48.
Mazei-Robison MS, Couch RS, Shelton RC, Stein MA, Blakely RD. Sequence variation in the human dopamine transporter gene in children with attention deficit hyperactivity disorder. Neuropharmacology. 2005;49:724–736.
Bowton E, Saunders C, Reddy IA, Campbell NG, Hamilton PJ, Henry LK, et al. SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking. Transl psychiatry. 2014;4:e464.
Shoaib A, Cepeda MS, Murray G, Ochs-Ross R Autism: Comorbidities and treatment patterns in the real world, a retrospective cohort study among children, adolescents and adults newly diagnosed with Autism. J Autism Dev Disord. 2021. e-pub ahead of print. https://doi.org/10.1007/s10803-021-05289-x.
Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, et al. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–7046.
Mergy MA, Gowrishankar R, Gresch PJ, Gantz SC, Williams J, Davis GL, et al. The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proc Natl Acad Sci USA. 2014;111:E4779–4788.
Davis GL, Stewart A, Stanwood GD, Gowrishankar R, Hahn MK, Blakely RD. Functional coding variation in the presynaptic dopamine transporter associated with neuropsychiatric disorders drives enhanced motivation and context-dependent impulsivity in mice. Behav Brain Res. 2018;337:61–69.
Gowrishankar R, Gresch PJ, Davis GL, Katamish RM, Riele JR, Stewart AM, et al. Region-specific regulation of presynaptic dopamine homeostasis by D2 autoreceptors shapes the in vivo impact of the neuropsychiatric disease-associated DAT variant Val559. J Neurosci: Off J Soc Neurosci. 2018;38:5302–5312.
Grunhage F, Schulze TG, Muller DJ, Lanczik M, Franzek E, Albus M, et al. Systematic screening for DNA sequence variation in the coding region of the human dopamine transporter gene (DAT1). Mol Psychiatry. 2000;5:275–282.
Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26:595–620.
McElroy SL. Bipolar disorders: special diagnostic and treatment considerations in women. CNS Spectr. 2004;9:5–18.
Stewart A, Davis GL, Gresch PJ, Katamish RM, Peart R, Rabil MJ, et al. Serotonin transporter inhibition and 5-HT2C receptor activation drive loss of cocaine-induced locomotor activation in DAT Val559 mice. Neuropsychopharmacology. 2019;44:994–1006.
Ragu Varman D, Subler MA, Windle JJ, Jayanthi LD, Ramamoorthy S. Novelty-induced hyperactivity and suppressed cocaine induced locomotor activation in mice lacking threonine 53 phosphorylation of dopamine transporter. Behav Brain Res. 2021;408:113267.
Owens WA, Williams JM, Saunders C, Avison MJ, Galli A, Daws LC. Rescue of dopamine transporter function in hypoinsulinemic rats by a D2 receptor-ERK-dependent mechanism. J Neurosci. 2012;32:2637–2647.
Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198.
MacPhail RC, Gollub LR. Independence of the effects of d-amphetamine and food deprivation or body weight on the food consumption of rats. Psychopharmacologia. 1974;34:163–173.
Moore S, Kenyon P. Atypical antipsychotics, clozapine and sulpiride do not antagonise amphetamine-induced stereotyped locomotion. Psychopharmacol (Berl). 1994;114:123–130.
Zachry JE, Nolan SO, Brady LJ, Kelly SJ, Siciliano CA, Calipari ES. Sex differences in dopamine release regulation in the striatum. Neuropsychopharmacology. 2021;46:491–499.
Bass AS, Robie NW. Stereoselectivity of S- and R-sulpiride for pre- and postsynaptic dopamine receptors in the canine kidney. J Pharm Exp Ther. 1984;229:67–71.
Ma GF, Raivio N, Sabria J, Ortiz J. Agonist and antagonist effects of aripiprazole on D(2)-like receptors controlling rat brain dopamine synthesis depend on the dopaminergic tone. Int J Neuropsychopharmacol. 2014;18:pyu046.
Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684.
Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B, et al. Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharm. 1992;225:331–337.
Chang PK, Chien KY, Chen JC. Dopamine transporter is downregulated and its association with chaperone protein Hsc70 is enhanced by activation of dopamine D3 receptor. Brain Res Bull. 2020;165:263–271.
Luis-Ravelo D, Fumagallo-Reading F, Castro-Hernandez J, Barroso-Chinea P, Afonso-Oramas D, Febles-Casquero A, et al. Prolonged dopamine D3 receptor stimulation promotes dopamine transporter ubiquitination and degradation through a PKC-dependent mechanism. Pharm Res. 2021;165:105434.
Castro-Hernandez J, Afonso-Oramas D, Cruz-Muros I, Salas-Hernandez J, Barroso-Chinea P, Moratalla R, et al. Prolonged treatment with pramipexole promotes physical interaction of striatal dopamine D3 autoreceptors with dopamine transporters to reduce dopamine uptake. Neurobiol Dis. 2015;74:325–335.
Chang PK, Yu L, Chen JC. Dopamine D3 receptor blockade rescues hyper-dopamine activity-induced deficit in novel object recognition memory. Neuropharmacology. 2018;133:216–223.
Chang PK, Chu J, Tsai YT, Lai YH, Chen JC. Dopamine D3 receptor and GSK3beta signaling mediate deficits in novel object recognition memory within dopamine transporter knockdown mice. J Biomed Sci. 2020;27:16.
Leo D, Sukhanov I, Zoratto F, Illiano P, Caffino L, Sanna F, et al. Pronounced hyperactivity, cognitive dysfunctions, and BDNF dysregulation in dopamine transporter knock-out rats. J Neurosci. 2018;38:1959–1972.
Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496.
Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem. 2006;281:32072–32080.
Shi X, McGinty JF. Extracellular signal-regulated mitogen-activated protein kinase inhibitors decrease amphetamine-induced behavior and neuropeptide gene expression in the striatum. Neuroscience. 2006;138:1289–1298.
Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20.
Cannon CM, Abdallah L, Tecott LH, During MJ, Palmiter RD. Dysregulation of striatal dopamine signaling by amphetamine inhibits feeding by hungry mice. Neuron. 2004;44:509–520.
Sotak BN, Hnasko TS, Robinson S, Kremer EJ, Palmiter RD. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005;1061:88–96.
Visser E, Matos MR, Mitrić M, Kramvis I, van der Loo RJ, Mansvelder HD, et al. Extinction of cocaine memory depends on a feed-forward inhibition circuit within the medial prefrontal cortex. Biological Psychiatry. 2021;91:1029–1038.
Lee Y, Kim H, Kim JE, Park JY, Choi J, Lee JE, et al. Excessive D1 dopamine receptor activation in the dorsal striatum promotes autistic-like behaviors. Mol Neurobiol. 2018;55:5658–5671.
Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A. et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551.
Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104.
Annett LE, McGregor A, Robbins TW. The effects of ibotenic acid lesions of the nucleus accumbens on spatial learning and extinction in the rat. Behav Brain Res. 1989;31:231–242.
Thifault S, Kremarik P, Lalonde R. Effects of bilateral electrolytic lesions of the medial nucleus accumbens on exploration and spatial learning. Arch Physiol Biochem. 1998;106:297–307.
Taghzouti K, Simon H, Herve D, Blanc G, Studler JM, Glowinski J, et al. Behavioural deficits induced by an electrolytic lesion of the rat ventral mesencephalic tegmentum are corrected by a superimposed lesion of the dorsal noradrenergic system. Brain Res. 1988;440:172–176.
Divac I, Wikmark RGE, Gade A. Spontaneous alternation in rats with lesions in the frontal lobes: an extension of the frontal lobe syndrome. Physiological Psychol. 1975;3:39–42.
Ferrazzo S, Gunduz-Cinar O, Stefanova N, Pollack GA, Holmes A, Schmuckermair C, et al. Increased anxiety-like behavior following circuit-specific catecholamine denervation in mice. Neurobiol Dis. 2019;125:55–66.
Poulin JF, Caronia G, Hofer C, Cui Q, Helm B, Ramakrishnan C, et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci. 2018;21:1260–1271.
Ahn JR, Lee I. Neural correlates of object-associated choice behavior in the perirhinal cortex of rats. J Neurosci. 2015;35:1692–1705.
Warburton EC, Brown MW. Neural circuitry for rat recognition memory. Behav Brain Res. 2015;285:131–139.
Balderas I, Moreno-Castilla P, Bermudez-Rattoni F. Dopamine D1 receptor activity modulates object recognition memory consolidation in the perirhinal cortex but not in the hippocampus. Hippocampus. 2013;23:873–878.
Bettis T, Jacobs LF. Sex differences in object recognition are modulated by object similarity. Behav Brain Res. 2012;233:288–292.
Wallace KJ, Hofmann HA. Equal performance but distinct behaviors: sex differences in a novel object recognition task and spatial maze in a highly social cichlid fish. Anim Cogn 2021;24:1057–1073.
Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007;26:2127–2136.
Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–477.
Chen R, Daining CP, Sun H, Fraser R, Stokes SL, Leitges M, et al. Protein kinase Cbeta is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J Neurochem. 2013;125:663–672.
Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, et al. Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol. 2006;16:415–420.
Mariggio MA, Palumbi R, Vinella A, Laterza R, Petruzzelli MG, Peschechera A, et al. DRD1 and DRD2 Receptor Polymorphisms: Genetic Neuromodulation of the Dopaminergic System as a Risk Factor for ASD, ADHD and ASD/ADHD Overlap. Front Neurosci. 2021;15:705890.
Reith MEA, Kortagere S, Wiers CE, Sun H, Kurian MA, Galli A, et al. The dopamine transporter gene SLC6A3: multidisease risks. Mol Psychiatry. 2021;27:1031–1046.
Chen S, Qian A, Tao J, Zhou R, Fu C, Yang C, et al. Different effects of the DRD4 genotype on intrinsic brain network connectivity strength in drug-naive children with ADHD and healthy controls. Brain Imaging Behav. 2021;16:464–475.
Mazei-Robison MS, Blakely RD. Expression studies of naturally occurring human dopamine transporter variants identifies a novel state of transporter inactivation associated with Val382Ala. Neuropharmacology. 2005;49:737–749.
Herborg F, Andreassen TF, Berlin F, Loland CJ, Gether U. Neuropsychiatric disease-associated genetic variants of the dopamine transporter display heterogeneous molecular phenotypes. J Biol Chem. 2018;293:7250–7262.
Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, et al. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol psychiatry. 2013;18:1315–1323.
Herborg F, Jensen KL, Tolstoy S, Arends NV, Posselt LP, Shekar A, et al. Identifying dominant-negative actions of a dopamine transporter variant in patients with parkinsonism and neuropsychiatric disease. JCI Insight. 2021;6:e151496.
Cartier E, Hamilton PJ, Belovich AN, Shekar A, Campbell NG, Saunders C, et al. Rare autism-associated variants implicate syntaxin 1 (STX1 R26Q) phosphorylation and the dopamine transporter (hDAT R51W) in dopamine neurotransmission and behaviors. EBioMedicine. 2015;2:135–146.
Hansen FH, Skjorringe T, Yasmeen S, Arends NV, Sahai MA, Erreger K, et al. Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J Clin Invest. 2014;124:3107–3120.
Campbell NG, Shekar A, Aguilar JI, Peng D, Navratna V, Yang D, et al. Structural, functional, and behavioral insights of dopamine dysfunction revealed by a deletion in SLC6A3. Proc Natl Acad Sci USA. 2019;116:3853–3862.
Bowton E, Saunders C, Erreger K, Sakrikar D, Matthies HJ, Sen N, et al. Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. J Neurosci. 2010;30:6048–6057.
Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359:693–697.
Acknowledgements
We wish to acknowledge the infrastructure support from Blakely laboratory staff members Matthew Gross, Alaina Tillman, and Zayna Gichi. The authors thank the Vanderbilt and Florida Atlantic University (FAU) Mouse Neurobehavioral Cores for support of behavioral experiments and neurochemical analyses. Financial support for this work derives from the Postdoctoral Training Program in Functional Neurogenomics MH065215 (AS), NARSAD Young Investigator Grant from the BBRF (AS), a Max Kade fellowship from the Austrian Academy of Sciences (FPM), and NIH awards MH107132 (GLD), 2P20GM104360 (RAV) and MH086530 (RDB). RP and AEW received grant support from the Office for Undergraduate Research and Inquiry (OURI) at FAU.
Author information
Authors and Affiliations
Contributions
AS and RDB conceived and designed experiments. FPM and RG performed slice biotinylation and pT53 pull down experiments. RG performed chronoamperometry. PJG performed surgeries and sample collection for microdialysis experiments and RMK performed HPLC analysis of dialysates. LBA performed the social interaction test. GLD performed the pERK immunoblotting experiment. AS performed all additional experiments with the assistance of RP, SES, KS, MJR, FAD, and AEW. RAV developed essential reagents. AS and RDB wrote the manuscript. M.K.H. aided in manuscript revision. All authors reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stewart, A., Mayer, F.P., Gowrishankar, R. et al. Behaviorally penetrant, anomalous dopamine efflux exposes sex and circuit dependent regulation of dopamine transporters. Mol Psychiatry 27, 4869–4880 (2022). https://doi.org/10.1038/s41380-022-01773-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01773-7