Abstract
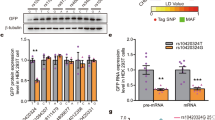
Inappropriate aggression in humans hurts the society, families and individuals. The genetic basis for aggressive behavior, however, remains largely elusive. In this study, we identified two rare missense variants in X-linked GRIA3 from male patients who showed syndromes featuring aggressive outbursts. Both G630R and E787G mutations in AMPA receptor GluA3 completely lost their ion channel functions. Furthermore, a guanine-repeat single nucleotide polymorphism (SNP, rs3216834) located in the first intron of human GRIA3 gene was found to regulate GluA3 expression with longer guanine repeats (rs3216834-10G/-11G) suppressing transcription compared to the shorter ones (-7G/-8G/-9G). Importantly, the distribution of rs3216834-10G/-11G was elevated in a male violent criminal sample from Chinese Han population. Using GluA3 knockout mice, we showed that the excitatory neurotransmission and neuronal activity in the medial prefrontal cortex (mPFC) was impaired. Expressing GluA3 back into the mPFC alleviated the aggressive behavior of GluA3 knockout mice, suggesting that the defects in mPFC explained, at least partially, the neural mechanisms underlying the aggressive behavior. Therefore, our study provides compelling evidence that dysfunction of AMPA receptor GluA3 promotes aggressive behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lorenz K. On aggression. 1st edn New York: Harcourt 1966.
Filley CM, Price BH, Nell V, Antoinette T, Morgan AS, Bresnahan JF, et al. Toward an understanding of violence: neurobehavioral aspects of unwarranted physical aggression: Aspen Neurobehavioral Conference consensus statement. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:1–14.
Lischinsky JE, Lin D. Neural mechanisms of aggression across species. Nat Neurosci. 2020;23:1317–28.
Waltes R, Chiocchetti AG, Freitag CM. The neurobiological basis of human aggression: A review on genetic and epigenetic mechanisms. Am J Med Genet B Neuropsychiatr Genet. 2016;171:650–75.
Fernandez-Castillo N, Cormand B. Aggressive behavior in humans: Genes and pathways identified through association studies. Am J Med Genet B Neuropsychiatr Genet. 2016;171:676–96.
Swann AC. Neuroreceptor mechanisms of aggression and its treatment. J Clin Psychiatry. 2003;64:26–35.
Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–46.
Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–62.
Unger EK, Burke KJ Jr., Yang CF, Bender KJ, Fuller PM, Shah NM. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 2015;10:453–62.
Zhou QG, Wu HY, Zhou H, Liu MY, Lee HW, Liu X, et al. Reactivation of Tert in the medial prefrontal cortex and hippocampus rescues aggression and depression of Tert(−/−) mice. Transl Psychiatry. 2016;6:e836.
Yang T, Yang CF, Chizari MD, Maheswaranathan N, Burke KJ Jr., Borius M, et al. Social control of hypothalamus-mediated male aggression. Neuron. 2017;95:955–700.
Stagkourakis S, Spigolon G, Williams P, Protzmann J, Fisone G, Broberger C. A neural network for intermale aggression to establish social hierarchy. Nat Neurosci. 2018;21:834–42.
Falkner AL, Wei D, Song A, Watsek LW, Chen I, Chen P, et al. Hierarchical representations of aggression in a hypothalamic-midbrain circuit. Neuron. 2020;106:637–48.
Tschida K, Michael V, Takatoh J, Han BX, Zhao S, Sakurai K, et al. A specialized neural circuit gates social vocalizations in the mouse. Neuron. 2019;103:459–72.
Manchanda SK, Poddar A, Saha S, Bhatia SC, Kumar VM, Nayar U. Predatory aggression induced by hypothalamic stimulation: modulation by midbrain periaqueductal gray (PAG). Neurobiol (Bp). 1995;3:405–17.
Takahashi A, Nagayasu K, Nishitani N, Kaneko S, Koide T. Control of intermale aggression by medial prefrontal cortex activation in the mouse. PLoS One. 2014;9:e94657.
Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–4.
Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by positron emission tomography. Biol Psychiatry. 1997;42:495–508.
Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, et al. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–6.
Cherepkova EV, Maksimov VV, Aftanas LI. Polymorphism of serotonin transporter gene in male subjects with antisocial behavior and MMA fighters. Transl Psychiatry. 2018;8:248.
Lewis AS, Pittenger ST, Mineur YS, Stout D, Smith PH, Picciotto MR. Bidirectional regulation of aggression in mice by hippocampal alpha-7 nicotinic acetylcholine receptors. Neuropsychopharmacology. 2018;43:1267–75.
Peng SX, Wang YY, Zhang M, Zang YY, Wu D, Pei J, et al. SNP rs10420324 in the AMPA receptor auxiliary subunit TARP gamma-8 regulates the susceptibility to antisocial personality disorder. Sci Rep. 2021;11:11997.
Brodkin ES, Goforth SA, Keene AH, Fossella JA, Silver LM. Identification of quantitative trait Loci that affect aggressive behavior in mice. J Neurosci. 2002;22:1165–70.
Adamczyk A, Mejias R, Takamiya K, Yocum J, Krasnova IN, Calderon J, et al. GluA3-deficiency in mice is associated with increased social and aggressive behavior and elevated dopamine in striatum. Behav Brain Res. 2012;229:265–72.
Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108.
Veroude K, Zhang-James Y, Fernandez-Castillo N, Bakker MJ, Cormand B, Faraone SV. Genetics of aggressive behavior: An overview. Am J Med Genet B Neuropsychiatr Genet. 2016;171B:3–43.
Odintsova VV, Roetman PJ, Ip HF, Pool R, Van der Laan CM, Tona KD, et al. Genomics of human aggression: current state of genome-wide studies and an automated systematic review tool. Psychiatr Genet. 2019;29:170–90.
Zhang-James Y, Fernandez-Castillo N, Hess JL, Malki K, Glatt SJ, Cormand B, et al. An integrated analysis of genes and functional pathways for aggression in human and rodent models. Mol Psychiatry. 2019;24:1655–67.
Sun JH, Chen J, Ayala Valenzuela FE, Brown C, Masser-Frye D, Jones M, et al. X-linked neonatal-onset epileptic encephalopathy associated with a gain-of-function variant p.R660T in GRIA3. PLoS Genet. 2021;17:e1009608.
Hamanaka K, Miyoshi K, Sun JH, Hamada K, Komatsubara T, Saida K, et al. Amelioration of a neurodevelopmental disorder by carbamazepine in a case having a gain-of-function GRIA3 variant. Hum Genet. 2022;141:283–93.
Rinaldi B, Ge YH, Freri E, Tucci A, Granata T, Estienne M, et al. Myoclonic status epilepticus and cerebellar hypoplasia associated with a novel variant in the GRIA3 gene. Neurogenetics. 2022;23:27–35.
Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–9.
Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–9.
Ho PA, Alonzo TA, Gerbing RB, Kuhn J, Pollard JA, Hirsch B, et al. The prognostic effect of high diagnostic WT1 gene expression in pediatric AML depends on WT1 SNP rs16754 status: report from the Children’s Oncology Group. Pediatr Blood Cancer. 2014;61:81–8.
Brym P, Malewski T, Starzynski R, Flisikowski K, Wojcik E, Rusc A, et al. Effect of new SNP within bovine prolactin gene enhancer region on expression in the pituitary gland. Biochem Genet. 2007;45:743–54.
Gupta RM, Hadaya J, Trehan A, Zekavat SM, Roselli C, Klarin D, et al. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell. 2017;170:522–33.
Xu Z, Taylor JA. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009;37:W600–605.
Kekedy-Nagy L, Sorensen KD, Ferapontova EE. Picomolar sensitive and SNP-selective “Off-On” hairpin genosensor based on structure-tunable redox indicator signals. Biosens Bioelectron. 2018;117:444–9.
Chaudhary S, Kaushik M, Ahmed S, Kukreti R, Kukreti S. Structural switch from hairpin to duplex/antiparallel G-quadruplex at single-nucleotide polymorphism (SNP) site of human apolipoprotein E (APOE) gene coding region. ACS Omega. 2018;3:3173–82.
Varshney D, Spiegel J, Zyner K, Tannahill D, Balasubramanian S. The regulation and functions of DNA and RNA G-quadruplexes. Nat Rev Mol Cell Biol. 2020;21:459–74.
Zhang M, Liu N, Chen H, Zhang N. Oxytocin receptor gene, childhood maltreatment and borderline personality disorder features among male inmates in China. BMC Psychiatry. 2020;20:332.
Biro L, Sipos E, Bruzsik B, Farkas I, Zelena D, Balazsfi D, et al. Task division within the prefrontal cortex: distinct neuron populations selectively control different aspects of aggressive behavior via the hypothalamus. J Neurosci. 2018;38:4065–75.
Hesse AN, Bevilacqua J, Shankar K, Reddi HV. Retrospective genotype-phenotype analysis in a 305 patient cohort referred for testing of a targeted epilepsy panel. Epilepsy Res. 2018;144:53–61.
Trivisano M, Santarone ME, Micalizzi A, Ferretti A, Dentici ML, Novelli A, et al. GRIA3 missense mutation is cause of an x-linked developmental and epileptic encephalopathy. Seizure. 2020;82:1–6.
Allen NM, Conroy J, Shahwan A, Lynch B, Correa RG, Pena SD, et al. Unexplained early onset epileptic encephalopathy: Exome screening and phenotype expansion. Epilepsia. 2016;57:e12–17.
Piard J, Bereau M, XiangWei W, Wirth T, Amsallem D, Buisson L, et al. The GRIA3 c.2477G > a variant causes an exaggerated startle reflex, chorea, and multifocal myoclonus. Mov Disord. 2020;35:1224–32.
Philips AK, Siren A, Avela K, Somer M, Peippo M, Ahvenainen M, et al. X-exome sequencing in Finnish families with intellectual disability–four novel mutations and two novel syndromic phenotypes. Orphanet J Rare Dis. 2014;9:49.
Jancic D, Seifuddin F, Zandi PP, Potash JB, Willour VL. Association study of X chromosome SNPs in attempted suicide. Psychiatry Res. 2012;200:1044–6.
Bodily PM, Fujimoto MS, Page JT, Clement MJ, Ebbert MT, Ridge PG, et al. A novel approach for multi-SNP GWAS and its application in Alzheimer’s disease. BMC Bioinforma. 2016;17:268.
Iamjan SA, Thanoi S, Watiktinkorn P, Reynolds GP, Nudmamud-Thanoi S. Genetic variation of GRIA3 gene is associated with vulnerability to methamphetamine dependence and its associated psychosis. J Psychopharmacol. 2018;32:309–15.
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31:827–32.
Chen J, Du Y, He X, Huang X, Shi YS. A convenient Cas9-based conditional knockout strategy for simultaneously targeting multiple genes in mouse. Sci Rep. 2017;7:517.
He XY, Li YJ, Kalyanaraman C, Qiu LL, Chen C, Xiao Q, et al. GluA1 signal peptide determines the spatial assembly of heteromeric AMPA receptors. Proc Natl Acad Sci USA. 2016;113:E5645–54.
Li YJ, Duan GF, Sun JH, Wu D, Ye C, Zang YY, et al. Neto proteins regulate gating of the kainate-type glutamate receptor GluK2 through two binding sites. J Biol Chem. 2019;294:17889–902.
Acknowledgements
We thank Dr. Zhengping Jia in University of Toronto for the generous gift of GluA3 KO mice. Fundings: This work is supported by grants from the National Key R&D Program of China (2019YFA0801603 to Y.S.S. and 2017YFA0105201 to C.Z.), the National Natural Science Foundation of China (32170951 and 91849112 to Y.S.S., 81901161 to J.C., 82171189 and 81971020 to J.J.Y., 31871032 to N.S. and 81901390 to N.L.), the Natural Science Foundation of Jiangsu Province (BE2019707 to Y.S.S.), Special Fund for Science and Technology Innovation Strategy of Guangdong Province (2021B0909050004 to Y.S.S.), the Fundamental Research Funds for the Central Universities (0903-14380029 to Y.S.S.), Strategic Priority Research Program of the Chinese Academy of Sciences (XDPB17 to NS), and Yunnan Applied Basic Research Projects (2019FA008 and 2019FJ003 to N.S.).
Author information
Authors and Affiliations
Contributions
SXP, MJ, and YYZ conducted animal behavioral and molecular experiments. JP, JW, NZ, XG, XX, and NL designed questionnaire and provided human samples. BR, ADD, AB, DM, and BG collected GRIA3 pathogenic variants in subjects referred for neurodevelopmental disorders. JC, SXP, YHG, and JHS conducted electrophysiological experiments. YYZ and YYS took care of the animals in the study. NS, CZ, and JJY provided critical reagents and supervised the study. YSS designed the study and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Peng, SX., Pei, J., Rinaldi, B. et al. Dysfunction of AMPA receptor GluA3 is associated with aggressive behavior in human. Mol Psychiatry 27, 4092–4102 (2022). https://doi.org/10.1038/s41380-022-01659-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01659-8