Abstract
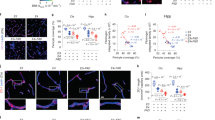
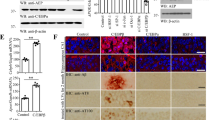
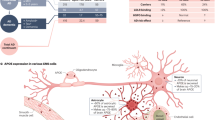
Atherosclerosis (ATH) and Alzheimer’s disease (AD) are both age-dependent inflammatory diseases, associated with infiltrated macrophages and vascular pathology and overlapping molecules. C/EBPβ, an Aβ or inflammatory cytokine-activated transcription factor, and AEP (asparagine endopeptidase) are intimately implicated in both ATH and AD; however, whether C/EBPβ/AEP signaling couples ATH to AD pathogenesis remains incompletely understood. Here we show that C/EBPβ/AEP pathway mediates ATH pathology and couples ATH to AD. Deletion of C/EBPβ or AEP from primary macrophages diminishes cholesterol load, and inactivation of this pathway reduces foam cell formation and lesions in aorta in ApoE−/− mice, fed with HFD (high-fat-diet). Knockout of ApoE from 3xTg AD mouse model augments serum LDL and increases lesion areas in the aorta. Depletion of C/EBPβ or AEP from 3xTg/ApoE−/− mice substantially attenuates these effects and elevates cerebral blood flow and vessel length, improving cognitive functions. Strikingly, knockdown of ApoE from the hippocampus of 3xTg mice decreases the cerebral blood flow and vessel length and aggravates AD pathologies, leading to cognitive deficits. Inactivation of C/EBPβ/AEP pathway alleviates these events and restores cognitive functions. Hence, our findings demonstrate that C/EBPβ/AEP signaling couples ATH to AD via mediating vascular pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Emini Veseli B, Perrotta P, De Meyer GRA, Roth L, Van der Donckt C, Martinet W, et al. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13.
Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–19.
Sato Y, Watanabe R, Uchiyama N, Ozawa N, Takahashi Y, Shirai R, et al. Inhibitory effects of vasostatin-1 against atherogenesis. Clin Sci. 2018;132:2493–507.
Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41.
Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–46.
Lathe R, Sapronova A, Kotelevtsev Y. Atherosclerosis and Alzheimer—diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatrics. 2014;14:36.
Kokjohn TA, Van Vickle GD, Maarouf CL, Kalback WM, Hunter JM, Daugs ID, et al. Chemical characterization of pro-inflammatory amyloid-beta peptides in human atherosclerotic lesions and platelets. Biochim Biophys Acta Mol Basis Dis. 2011;1812:1508–14.
Li L, Cao D, Garber DW, Kim H, Fukuchi K-I. Association of aortic atherosclerosis with cerebral β-amyloidosis and learning deficits in a mouse model of Alzheimer’s disease. Am J Pathol. 2003;163:2155–64.
Tibolla G, Norata GD, Meda C, Arnaboldi L, Uboldi P, Piazza F, et al. Increased atherosclerosis and vascular inflammation in APP transgenic mice with apolipoprotein E deficiency. Atherosclerosis. 2010;210:78–87.
Van De Parre TJ, Guns PJ, Fransen P, Martinet W, Bult H, Herman AG, et al. Attenuated atherogenesis in apolipoprotein E-deficient mice lacking amyloid precursor protein. Atherosclerosis. 2011;216:54–58.
Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004;24:1006–14.
Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–30.
Breslow JL. Mouse Models of Atherosclerosis. Science. 1996;272:685.
Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Investig. 1994;93:1885–93.
Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA. 1992;89:4471–5.
Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–53.
Silvestre-Roig C, de Winther MP, Weber C, Daemen MJ, Lutgens E, Soehnlein O. Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circulation Res. 2014;114:214–26.
Chen JM, Dando PM, Rawlings ND, Brown MA, Young NE, Stevens RA, et al. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J Biol Chem. 1997;272:8090–8.
Zhang Z, Song M, Liu X, Kang SS, Kwon IS, Duong DM, et al. Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nat Med. 2014;20:1254–62.
Zhang Z, Song M, Liu X, Su Kang S, Duong DM, Seyfried NT, et al. Delta-secretase cleaves amyloid precursor protein and regulates the pathogenesis in Alzheimer’s disease. Nat Commun. 2015;6:8762.
Wu Z, Liu X, Cheng L, Ye K. Delta-secretase triggers Alzheimer’s disease pathologies in wild-type hAPP/hMAPT double transgenic mice. Cell Death Dis. 2020;11:1058.
Zhang Z, Obianyo O, Dall E, Du Y, Fu H, Liu X, et al. Inhibition of delta-secretase improves cognitive functions in mouse models of Alzheimer’s disease. Nat Commun. 2017;8:14740.
Mattock KL, Gough PJ, Humphries J, Burnand K, Patel L, Suckling KE, et al. Legumain and cathepsin-L expression in human unstable carotid plaque. Atherosclerosis. 2010;208:83–89.
Ammirati E, Fogacci F. Clinical relevance of biomarkers for the identification of patients with carotid atherosclerotic plaque: Potential role and limitations of cysteine protease legumain. Atherosclerosis. 2017;257:248–9.
Lunde NN, Holm S, Dahl TB, Elyouncha I, Sporsheim B, Gregersen I, et al. Increased levels of legumain in plasma and plaques from patients with carotid atherosclerosis. Atherosclerosis. 2017;257:216–23.
Ozawa N, Sato Y, Mori Y, Masuda H, Yamane M, Yamamoto Y, et al. Legumain promotes atherosclerotic vascular remodeling. Int J Mol Sci. 2019;20:2195.
Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, et al. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45:1108–17.
Schroeder-Gloeckler JM, Rahman SM, Janssen RC, Qiao L, Shao J, Roper M, et al. CCAAT/enhancer-binding protein beta deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J Biol Chem. 2007;282:15717–29.
Wang ZH, Gong K, Liu X, Zhang Z, Sun X, Wei ZZ, et al. C/EBPβ regulates delta-secretase expression and mediates pathogenesis in mouse models of Alzheimer’s disease. Nat Commun. 2018;9:1784.
Wang H, Liu X, Chen S, Ye K. Spatiotemporal activation of the C/EBPβ/δ-secretase axis regulates the pathogenesis of Alzheimer’s disease. Proc Natl Acad Sci USA. 2018;115:E12427.
Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–41.
Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994;14:141–7.
Rahman SM, Baquero KC, Choudhury M, Janssen RC, de la Houssaye BA, Sun M, et al. C/EBPβ in bone marrow is essential for diet induced inflammation, cholesterol balance, and atherosclerosis. Atherosclerosis. 2016;250:172–9.
Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–82.
Wang ZH, Xia Y, Liu P, Liu X, Edgington-Mitchell L, Lei K, et al. ApoE4 activates C/EBPβ/δ-secretase with 27-hydroxycholesterol, driving the pathogenesis of Alzheimer’s disease. Prog Neurobiol. 2021;202:102032.
Shirahama-Noda K, Yamamoto A, Sugihara K, Hashimoto N, Asano M, Nishimura M, et al. Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice*. J Biol Chem. 2003;278:33194–9.
Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;83:1–14.
Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–61.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74.
Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPbeta in female reproduction. Genes Dev. 1997;11:2153–62.
Gupta A, Iadecola C. Impaired Aβ clearance: a potential link between atherosclerosis and Alzheimer’s disease. Front Aging Neurosci. 2015;7:115.
Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–62.
Getz GS, Reardon CA. ApoE knockout and knockin mice: the history of their contribution to the understanding of atherogenesis. J Lipid Res. 2016;57:758–66.
Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, et al. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Investig. 1999;103:1579–86.
Zambón D, Quintana M, Mata P, Alonso R, Benavent J, Cruz-Sánchez F, et al. Higher incidence of mild cognitive impairment in familial hypercholesterolemia. Am J Med. 2010;123:267–74.
Xia Y, Wang ZH, Zhang J, Liu X, Yu SP, Ye KX, et al. C/EBPβ is a key transcription factor for APOE and preferentially mediates ApoE4 expression in Alzheimer’s disease. Mol Psychiatry. 2020;26:6002–22.
Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–96.
Roher AE, Debbins JP, Malek-Ahmadi M, Chen K, Pipe JG, Maze S, et al. Cerebral blood flow in Alzheimer’s disease. Vasc Health Risk Manag. 2012;8:599–611.
Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis. 2002;9:61–8.
Zaghi J, Goldenson B, Inayathullah M, Lossinsky AS, Masoumi A, Avagyan H, et al. Alzheimer disease macrophages shuttle amyloid-beta from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol. 2009;117:111–24.
Lee PH, Bang OY, Hwang EM, Lee JS, Joo US, Mook-Jung I, et al. Circulating beta amyloid protein is elevated in patients with acute ischemic stroke. J Neural Transm. 2005;112:1371–9.
Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. β-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–71.
Townsend K, Obregon D, Quadros A, Patel N, Volmar C, Paris D, et al. Proinflammatory and vasoactive effects of Aβ in the cerebrovasculature. Annals of the New York Academy of Sciences. 2002;977:65–76.
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–21.
Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, et al. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:15233–8.
Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, et al. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J Neurosci. 2003;23:7889.
Nilsson LN, Arendash GW, Leighty RE, Costa DA, Low MA, Garcia MF, et al. Cognitive impairment in PDAPP mice depends on ApoE and ACT-catalyzed amyloid formation. Neurobiol Aging. 2004;25:1153–67.
Yamada M. Risk factors for cerebral amyloid angiopathy in the elderly. Ann N Y Acad Sci. 2002;977:37–44.
Burns MP, Noble WJ, Olm V, Gaynor K, Casey E, LaFrancois J, et al. Co-localization of cholesterol, apolipoprotein E and fibrillar Aβ in amyloid plaques. Mol Brain Res. 2003;110:119–25.
Puglielli L, Friedlich AL, Setchell KD, Nagano S, Opazo C, Cherny RA, et al. Alzheimer disease beta-amyloid activity mimics cholesterol oxidase. J Clin Investig. 2005;115:2556–63.
Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28.
Vaya J, Aviram M, Mahmood S, Hayek T, Grenadir E, Hoffman A, et al. Selective distribution of oxysterols in atherosclerotic lesions and human plasma lipoproteins. Free Radic Res. 2001;34:485–97.
van den Kommer TN, Dik MG, Comijs HC, Fassbender K, Lütjohann D, Jonker C. Total cholesterol and oxysterols: Early markers for cognitive decline in elderly? Neurobiol Aging. 2009;30:534–45.
Geifman N, Brinton RD, Kennedy RE, Schneider LS, Butte AJ. Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer’s disease. Alzheimer’s Res Ther. 2017;9:10.
McGuinness B, Passmore P. Can statins prevent or help treat Alzheimer’s disease? J Alzheimer’s Dis. 2010;20:925–33.
Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis. The ASTEROID Trial. JAMA. 2006;295:1556–65.
Ballantyne CM, Raichlen JS, Nicholls SJ, Erbel R, Tardif JC, Brener SJ, et al. Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation. 2008;117:2458–66.
Acknowledgements
This work is supported by a grant from the National Institute of Health (RO1, AG065177) to KY. This study was supported in part by the Rodent Behavioral Core (RBC), which is subsidized by the Emory University School of Medicine and is one of the Emory Integrated Core Facilities. Additional support was provided by the Viral Vector Core of the Emory Neuroscience NINDS Core Facilities (P30NS055077). Further support was provided by the Georgia Clinical & Translational Science Alliance of the National Institutes of Health under Award Number UL1TR002378.
Author information
Authors and Affiliations
Contributions
KY conceived the project, designed the experiments, analyzed the data and wrote the manuscript. JL and GC designed and performed most of the experiments and analyzed the data. XL prepared primary macrophages and assisted with in vivo and in vitro experiments. ZZW, SPY and QC assisted with data analysis and interpretation and critically read the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liao, J., Chen, G., Liu, X. et al. C/EBPβ/AEP signaling couples atherosclerosis to the pathogenesis of Alzheimer’s disease. Mol Psychiatry 27, 3034–3046 (2022). https://doi.org/10.1038/s41380-022-01556-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01556-0
This article is cited by
-
Involvement of Fgf2-mediated tau protein phosphorylation in cognitive deficits induced by sevoflurane in aged rats
Molecular Medicine (2024)
-
Sepsis exacerbates Alzheimer’s disease pathophysiology, modulates the gut microbiome, increases neuroinflammation and amyloid burden
Molecular Psychiatry (2023)