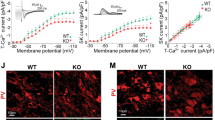
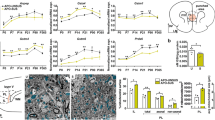
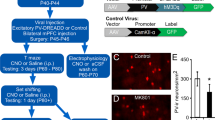
Abstract
Recent evidence showed thalamic abnormalities in schizophrenia involving disruptions to the parvalbumin neurons in the thalamic reticular nucleus (TRN). However, their functional consequences, as well as a potential linkage to oxidative stress, are unclear. The TRN is posited to gate prefrontal control of dopamine neuron activity in the ventral tegmental area (VTA). Thus, we hypothesized that schizophrenia-related TRN abnormalities might contribute to dopamine dysregulation, a well-known feature of the disorder. To test this, in adult rats exposed prenatally to methylazoxymethanol acetate (MAM rats), oxidative impairments to the parvalbumin neurons in the anterior TRN were assessed by immunohistochemistry. Using in vivo electrophysiology, we investigated whether inactivation of the prefrontal cortex would produce differential effects on VTA dopamine neurons in MAM rats. We show that MAM rats displayed reduced markers of parvalbumin and wisteria floribunda agglutinin-labeled perineuronal nets, correlating with increased markers of oxidative stress (8-oxo-7, 8-dihydro-20-deoxyguanosine, and 3-nitrotyrosine). Moreover, MAM rats displayed heightened baseline and abnormal prefrontal control of VTA dopamine neuron activity, as tetrodotoxin-induced inactivation of the infralimbic prefrontal cortex decreased the dopamine population activity, contrary to the normal increase in controls. Such dopamine neuron dysregulation was recapitulated by enzymatic perineuronal net digestion in the TRN of normal rats. Furthermore, juvenile (postnatal day 11–25) antioxidant treatment (N-acetyl-cysteine, 900 mg/L drinking water) prevented all these impairments in MAM rats. Our findings suggest that early accumulation of oxidative stress in the TRN may shape the later onset of schizophrenia pathophysiology, highlighting redox regulation as a potential target for early intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–20.
Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA, et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry. 2017;22:936–43.
Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–40.
Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S. et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–83.
Perkins DO, Jeffries CD, Do KQ. Potential roles of redox dysregulation in the development of schizophrenia. Biol Psychiatry. 2020;88:326−336.
Kann O, Papageorgiou IE, Draguhn A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab. 2014;34:1270–82.
Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–30.
Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–9.
Emiliani FE, Sedlak TW, Sawa A. Oxidative stress and schizophrenia: recent breakthroughs from an old story. Curr Opin Psychiatry. 2014;27:185–90.
Kulak A, Steullet P, Cabungcal J-H, Werge T, Ingason A, Cuenod M, et al. Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models. Antioxid Redox Signal. 2013;18:1428–43.
Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci USA 2007;104:16621–6.
Kulak A, Cuenod M, Do KQ. Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behavioural Brain Res. 2012;226:563–70.
Cabungcal J-H, Preissmann D, Delseth C, Cuénod M, Do KQ, Schenk F. Transitory glutathione deficit during brain development induces cognitive impairment in juvenile and adult rats: relevance to schizophrenia. Neurobiol Dis. 2007;26:634–45.
Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–8.
Conus P, Seidman LJ, Fournier M, Xin L, Cleusix M, Baumann PS, et al. N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. Schizophrenia Bull. 2018;44:317–27.
Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15:485.
Steullet P. Thalamus-related anomalies as candidate mechanism-based biomarkers for psychosis. Schizophr Res. 2019;226:147−157.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007;445:168–76.
Csillik B, Mihály A, Krisztin-Péva B, Chadaide Z, Samsam M, Knyihár-Csillik E, et al. GABAergic parvalbumin-immunoreactive large calyciform presynaptic complexes in the reticular nucleus of the rat thalamus. J Chem Neuroanat. 2005;30:17–26.
Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. A developmental redox dysregulation leads to spatio-temporal deficit of parvalbumin neuron circuitry in a schizophrenia mouse model. Schizophr Res. 2019;213:96–106.
Steullet P, Cabungcal J-H, Bukhari SA, Ardelt MI, Pantazopoulos H, Hamati F, et al. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry. 2018;23:2057–65.
Crabtree JW. Functional diversity of thalamic reticular subnetworks. Front Syst Neurosci. 2018;12:41.
Krol A, Wimmer RD, Halassa MM, Feng G. Thalamic reticular dysfunction as a circuit endophenotype in neurodevelopmental disorders. Neuron 2018;98:282–95.
Pratt JA, Morris BJ. The thalamic reticular nucleus: a functional hub for thalamocortical network dysfunction in schizophrenia and a target for drug discovery. J Psychopharmacol. 2015;29:127–37.
Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37:306–15.
You Q-L, Luo Z-c, Luo Z-Y, Kong Y, Li Z-l, Yang J-M, et al. Involvement of the thalamic reticular nucleus in prepulse inhibition of acoustic startle. Transl Psychiatry. 2021;11:241.
Richard EA, Khlestova E, Nanu R, Lisman JE. Potential synergistic action of 19 schizophrenia risk genes in the thalamus. Schizophrenia Res. 2017;180:64–9.
Tomitaka S, Tomitaka M, Tolliver BK, Sharp FR. Bilateral blockade of NMDA receptors in anterior thalamus by dizocilpine (MK‐801) injures pyramidal neurons in rat retrosplenial cortex. Eur J Neurosci. 2000;12:1420–30.
Sharp FR, Tomitaka M, Bernaudin M, Tomitaka S. Psychosis: pathological activation of limbic thalamocortical circuits by psychomimetics and schizophrenia? Trends Neurosci. 2001;24:330–4.
Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology 2003;28:265–75.
Steullet P, Cabungcal J-H, Kulak A, Kraftsik R, Chen Y, Dalton TP, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–58.
Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–32.
Grace AA. Dopamine system dysregulation by the hippocampus: Implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62:1342–8.
Lieberman JA, Girgis RR, Brucato G, Moore H, Provenzano F, Kegeles L, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23:1764–72.
Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67.
Patton MH, Bizup BT, Grace AA. The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci. 2013;33:16865–73.
Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 2004;51:32–58.
Zimmerman EC, Grace AA. Prefrontal cortex modulates firing pattern in the nucleus reuniens of the midline thalamus via distinct corticothalamic pathways. Eur J Neurosci. 2018;48:3255–72.
Zimmerman EC, Grace AA. The nucleus reuniens of the midline thalamus gates prefrontal-hippocampal modulation of ventral tegmental area dopamine neuron activity. J Neurosci. 2016;36:8977–84.
Modinos G, Allen P, Grace AA, McGuire P. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38:129–38.
Sawa A, Seidman LJ. Is prophylactic psychiatry around the corner? Combating adolescent oxidative stress for adult psychosis and schizophrenia. Neuron 2014;83:991–3.
Perez SM, Donegan JJ, Lodge DJ. Effect of estrous cycle on schizophrenia-like behaviors in MAM exposed rats. Behav Brain Res. 2019;362:258–65.
Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–64.
Cabungcal J-H, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron 2014;83:1073–84.
das Neves Duarte JM, Kulak A, Gholam-Razaee MM, Cuenod M, Gruetter R, Do KQ. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry. 2012;71:1006–14.
Cabungcal J-H, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry. 2013;73:574–82.
Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–30.
Klein PM, Lu AC, Harper ME, McKown HM, Morgan JD, Beenhakker MP. Tenuous inhibitory GABAergic signaling in the reticular thalamus. J Neurosci. 2018;38:1232–48.
Balmer TS. Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro 2016;3:4.
Moreines JL, Owrutsky ZL, Grace AA. Involvement of infralimbic prefrontal cortex but not lateral habenula in dopamine attenuation after chronic mild stress. Neuropsychopharmacology 2017;42:904–13.
Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proceedings of the National Academy of Sciences. 2013;110:9130–5.
Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–30.
Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–54.
Lodge DJ. The MAM rodent model of schizophrenia. Current protocols in neuroscience. 2013;63:9–43.
Do KQ, Cuenod M, Hensch TK. Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophrenia Bull. 2015;41:835–46.
Reyes-Madrigal F, León-Ortiz P, Mao X, Mora-Durán R, Shungu DC, de la Fuente-Sandoval C. Striatal glutathione in first-episode psychosis patients measured in vivo with proton magnetic resonance spectroscopy. Arch Med Res. 2019;50:207–13.
Das TK, Javadzadeh A, Dey A, Sabesan P, Théberge J, Radua J, et al. Antioxidant defense in schizophrenia and bipolar disorder: a meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;91:94–102.
Do KQ, Trabesinger AH, Kirsten-Krüger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–8.
Kumar J, Liddle EB, Fernandes CC, Palaniyappan L, Hall EL, Robson SE, et al. Glutathione and glutamate in schizophrenia: a 7T MRS study. Molecular Psychiatry. 2020;25:873–82.
Nucifora L, Tanaka T, Hayes L, Kim M, Lee B, Matsuda T, et al. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl Psychiatry 2017;7:e1215–e.
Wang AM, Pradhan S, Coughlin JM, Trivedi A, DuBois SL, Crawford JL, et al. Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiatry. 2019;76:314–23.
Fonnum F, Lock E. The contributions of excitotoxicity, glutathione depletion and DNA repair in chemically induced injury to neurones: exemplified with toxic effects on cerebellar granule cells. J Neurochem. 2004;88:513–31.
Gomes FV, Zhu X, Grace AA. Stress during critical periods of development and risk for schizophrenia. Schizophrenia Res. 2019;213:107–13.
Möller M, Du Preez JL, Emsley R, Harvey BH. Isolation rearing-induced deficits in sensorimotor gating and social interaction in rats are related to cortico-striatal oxidative stress, and reversed by sub-chronic clozapine administration. Eur Neuropsychopharmacol. 2011;21:471–83.
Schiavone S, Colaianna M, Curtis L. Impact of early life stress on the pathogenesis of mental disorders: relation to brain oxidative stress. Curr Pharm Des. 2015;21:1404–12.
Zimmerman EC, Bellaire M, Ewing SG, Grace AA. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology 2013;38:2131–9.
Phensy A, Duzdabanian HE, Brewer S, Panjabi A, Driskill C, Berz A, et al. Antioxidant treatment with N-acetyl cysteine prevents the development of cognitive and social behavioral deficits that result from perinatal ketamine treatment. Front Behav Neurosci. 2017;11:106.
Otte D-M, Sommersberg B, Kudin A, Guerrero C, Albayram Ö, Filiou MD, et al. N-acetyl cysteine treatment rescues cognitive deficits induced by mitochondrial dysfunction in G72/G30 transgenic mice. Neuropsychopharmacology 2011;36:2233–43.
Meister A. Glutathione biosynthesis and its inhibition. Methods Enzymol. 1995;252:26–30.
Majak K, Berdel B, Kowiański P, Dziewiatkowski J, Lipowska M, Moryś J. Parvalbumin immunoreactivity changes in the thalamic reticular nucleus during the maturation of the rat’s brain. Folia Neuropathol. 1998;36:7–14.
Mitrofanis J. Patterns of antigenic expression in the thalamic reticular nucleus of developing rats. J Comp Neurol. 1992;320:161–81.
Hou G, Smith AG, Zhang ZW. Lack of intrinsic GABAergic connections in the thalamic reticular nucleus of the mouse. J Neurosci: Off J Soc Neurosci. 2016;36:7246–52.
Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–61.
Halassa MM, Acsády L. Thalamic inhibition: diverse sources, diverse scales. Trends Neurosci. 2016;39:680–93.
Cornwall J, Cooper J, Phillipson O. Projections to the rostral reticular thalamic nucleus in the rat. Exp Brain Res. 1990;80:157–71.
Kolmac CI, Mitrofanis J. Organisation of the reticular thalamic projection to the intralaminar and midline nuclei in rats. J Comp Neurol. 1997;377:165–78.
Varela C, Kumar S, Yang JY, Wilson MA. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct. 2014;219:911–29.
McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480:115–42.
Sherman, S. & Guillery, R. Functional Connections of Cortical Areas: A New View from the Thalamus. Chapter 4, Classification of Afferents in Thalamus and Cortex; Cambridge, MA: The MIT Press. 2013. p.83–118.
Paz JT, Bryant AS, Peng K, Fenno L, Yizhar O, Frankel WN, et al. A new mode of corticothalamic transmission revealed in the Gria4(−/−) model of absence epilepsy. Nat Neurosci. 2011;14:1167–73.
Crandall SR, Cruikshank SJ, Connors BW. A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron 2015;86:768–82.
Warren RA, Agmon A, Jones EG. Oscillatory synaptic interactions between ventroposterior and reticular neurons in mouse thalamus in vitro. J Neurophysiol. 1994;72:1993–2003.
Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2016;79:1016–25.
Pantazopoulos H, Woo T-UW, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–66.
Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, et al. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52:751–62.
Shah A, Lodge DJ. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry 2013;3:e215-e.
Alemán‐Gómez Y, Najdenovska E, Roine T, Fartaria MJ, Canales‐Rodríguez EJ, Rovó Z, et al. Partial‐volume modeling reveals reduced gray matter in specific thalamic nuclei early in the time course of psychosis and chronic schizophrenia. Hum Brain Mapp. 2020;41:4041–61.
Acknowledgements
This work is supported by the National Institutes of Health (NIH MH57440 to AAG). We thank Niki MacMurdo and Dr. Rudolf Kraftsik for the technical assistance. We acknowledge funding support from the National Center of Competence in Research (NCCR) “SYNAPSY—The Synaptic Bases of Mental Diseases” from the Swiss National Science Foundation (n° 51AU40_125759 to KQD), Damm-Etienne Foundation, and Alamaya Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AAG has received funds from Lundbeck, Pfizer, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, Alkermes, Newron, Takeda, Concert, and Minerva. KQD received an investigator-initiated research grant from Boehringer Ingelheim outside the current study. No other disclosures were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhu, X., Cabungcal, JH., Cuenod, M. et al. Thalamic reticular nucleus impairments and abnormal prefrontal control of dopamine system in a developmental model of schizophrenia: prevention by N-acetylcysteine. Mol Psychiatry 26, 7679–7689 (2021). https://doi.org/10.1038/s41380-021-01198-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01198-8
This article is cited by
-
Net gain and loss: influence of natural rewards and drugs of abuse on perineuronal nets
Neuropsychopharmacology (2023)
-
Caught in vicious circles: a perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia
Molecular Psychiatry (2022)
-
Developmental oxidative stress leads to T-type Ca2+ channel hypofunction in thalamic reticular nucleus of mouse models pertinent to schizophrenia
Molecular Psychiatry (2022)
-
Hippocampal circuit dysfunction in psychosis
Translational Psychiatry (2022)
-
Time of exposure to social defeat stress during childhood and adolescence and redox dysregulation on long-lasting behavioral changes, a translational study
Translational Psychiatry (2022)