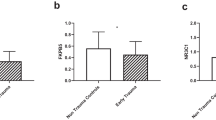
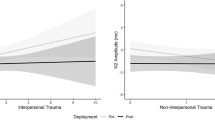
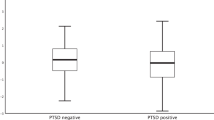
Abstract
Childhood adversity (CA) may alter reactivity to stress throughout life, increasing risk for psychiatric and medical morbidity, yet long-term correlates of milder CA levels among high functioning healthy adolescents are less studied. The current study examined the prevalence and impact of CA exposure among a cohort of healthy motivated elite parachute unit volunteers, prospectively assessed at rest and at the height of an intensive combat-simulation exposure. We found significantly reduced gene expression levels in resting mononuclear cell nuclear receptor, subfamily 3, member 1 (NR3C1), and its transactivator spindle and kinetochore-associated protein 2 (SKA2), that predict blunted cortisol reactivity to combat-simulation stress among CA exposed adolescents. Long-term alterations in endocrine immune indices, subjective distress, and executive functions persist among healthy high functioning adolescents following milder CA exposure, and may promote resilience or vulnerability to later real-life combat exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
van Bodegom M, Homberg JR, Henckens M. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci. 2017;11:87.
Raymond C, Marin MF, Majeur D, Lupien S. Early child adversity and psychopathology in adulthood: HPA axis and cognitive dysregulations as potential mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:152–60.
Rod NH, Bengtsson J, Budtz-Jorgensen E, Clipet-Jensen C, Taylor-Robinson D, Andersen AN, et al. Trajectories of childhood adversity and mortality in early adulthood: a population-based cohort study. Lancet. 2020;396:489–97.
Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e66.
Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37:268–77.
Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 2016;17:652–66.
Fritz J, de Graaff AM, Caisley H, van Harmelen AL, Wilkinson PO. A systematic review of amenable resilience factors that moderate and/or mediate the relationship between childhood adversity and mental health in young people. Front Psychiatry. 2018;9:230.
Holtge J, McGee SL, Maercker A, Thoma MV. Childhood adversities and thriving skills: sample case of older swiss former indentured child laborers. Am J Geriatr Psychiatry: Off J Am Assoc Geriatr Psychiatry 2018;26:886–95.
Woodward C, Joseph S. Positive change processes and post-traumatic growth in people who have experienced childhood abuse: understanding vehicles of change. Psychol Psychother. 2003;76:267–83.
Palma-Gudiel H, Cordova-Palomera A, Leza JC, Fananas L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: a critical review. Neurosci Biobehav Rev. 2015;55:520–35.
Bunea IM, Szentagotai-Tatar A, Miu AC. Early-life adversity and cortisol response to social stress: a meta-analysis. Transl Psychiatry 2017;7:1274.
Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, et al. Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. The TRAILS study. Psychoneuroendocrinology. 2012;37:1439–47.
Romens SE, McDonald J, Svaren J, Pollak SD. Associations between early life stress and gene methylation in children. Child Dev 2015;86:303–9.
Rice L, Waters CE, Eccles J, Garside H, Sommer P, Kay P, et al. Identification and functional analysis of SKA2 interaction with the glucocorticoid receptor. J Endocrinol. 2008;198:499–509.
Guintivano J, Brown T, Newcomer A, Jones M, Cox O, Maher BS, et al. Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. Am J Psychiatry. 2014;171:1287–96.
Boks MP, Rutten BP, Geuze E, Houtepen LC, Vermetten E, Kaminsky Z, et al. SKA2 methylation is involved in cortisol stress reactivity and predicts the development of post-traumatic stress disorder (PTSD) after military deployment. Neuropsychopharmacology. 2016;41:1350–6.
Kalla C, Goltser-Dubner T, Ben-Yehuda A, Itzhar N, Mirman A, Benarroch F, et al. Childhood adversity impact on elite army cadets coping with combat training stress. 2021. Submitted for publication.
Mirman A, Bick AS, Kalla C, Canetti L, Segman R, Dan R, et al. The imprint of childhood adversity on emotional processing in high functioning young adults. Hum Brain Mapp. 2021;42:615–25.
Bernstein DP, Fink L. Childhood trauma questionnaire: aretrospective self-report/ Manual. San Antonio, TX: The Psychological Corporation; 1998.
Osorio FL, Loureiro SR, Hallak JEC, Machado-de-Sousa JP, Ushirohira JM, Baes CVW, et al. Clinical validity and intrarater and test-retest reliability of the Structured Clinical Interview for DSM-5—Clinician Version (SCID-5-CV). Psychiatry Clin Neurosci. 2019;73:754–60.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71.
Roth RM, Isquith, PK, Gioia GA. Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A). Lutz, FL: Psychological Assessment Resources, Inc; 2005.
Spielberger CD, Gorsuch, RL., Lushene PR, Vagg PR, Jacobs AG. Manual for the state-trait anxiety inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press, Inc.; 1983.
Shalev APD, Kala C, Goltser-Dubner T, Benarroch F, Geisser R, Ben Yehuda A, et al. Altered cortisol response to combat training among elite parachute trainees with early childhood adversity. In: The International Society For Biological Psychiatry (ISBP) Annual Conference May 10, 2018 New York 2018.
Segman RH, Goltser-Dubner T, Weiner I, Canetti L, Galili-Weisstub E, Milwidsky A, et al. Blood mononuclear cell gene expression signature of postpartum depression. Mol Psychiatry. 2010;15:93–100.
Chang HB, Munroe S, Gray K, Porta G, Douaihy A, Marsland A, et al. The role of substance use, smoking, and inflammation in risk for suicidal behavior. J Affect Disord 2019;243:33–41.
Deighton S, Neville A, Pusch D, Dobson K. Biomarkers of adverse childhood experiences: a scoping review. Psychiatry Res. 2018;269:719–32.
Conradt E, Abar B, Lester BM, LaGasse LL, Shankaran S, Bada H, et al. Cortisol reactivity to social stress as a mediator of early adversity on risk and adaptive outcomes. Child Dev. 2014;85:2279–98.
Kuras YI, Assaf N, Thoma MV, Gianferante D, Hanlin L, Chen X, et al. Blunted diurnal cortisol activity in healthy adults with childhood adversity. Front Hum Neurosci. 2017;11:574.
Goldman-Mellor S, Hamer M, Steptoe A. Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology. 2012;37:1755–68.
O’Connor DB, Green JA, Ferguson E, O’Carroll RE, O’Connor RC. Effects of childhood trauma on cortisol levels in suicide attempters and ideators. Psychoneuroendocrinology. 2018;88:9–16.
O’Connor DB, Green JA, Ferguson E, O’Carroll RE, O’Connor RC. Cortisol reactivity and suicidal behavior: Investigating the role of hypothalamic-pituitary-adrenal axis responses to stress in suicide attempters and ideators. Psychoneuroendocrinology. 2017;75:183–91.
O’Connor DB, Ferguson E, Green JA, O’Carroll RE, O’Connor RC. Cortisol levels and suicidal behavior: a meta-analysis. Psychoneuroendocrinology 2016;63:370–9.
Young R. Trauma, attempted suicide, and morning cortisol in a community sample of adolescents. J Trauma Stress. 2010;23:288–91.
Gadek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharm Rep. 2013;65:1655–62.
McLaughlin KA, Koenen KC, Bromet EJ, Karam EG, Liu H, Petukhova M, et al. Childhood adversities and post-traumatic stress disorder: evidence for stress sensitisation in the World Mental Health Surveys. Br J Psychiatry. 2017;211:280–8.
Pesonen AK, Raikkonen K. The lifespan consequences of early life stress. Physiol Behav. 2012;106:722–7.
Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–73.
DePierro J, Lepow L, Feder A, Yehuda R. Translating molecular and neuroendocrine findings in posttraumatic stress disorder and resilience to novel therapies. Biol Psychiatry. 2019;86:454–63.
McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–7.
Watkeys OJ, Kremerskothen K, Quide Y, Fullerton JM, Green MJ. Glucocorticoid receptor gene (NR3C1) DNA methylation in association with trauma, psychopathology, transcript expression, or genotypic variation: A systematic review. Neurosci Biobehav Rev. 2018;95:85–122.
Amos T, Stein DJ, Ipser JC. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2014:1–53.
Resnick HS, Yehuda R, Pitman RK, Foy DW. Effect of previous trauma on acute plasma cortisol level following rape. Am J Psychiatry. 1995;152:1675–7.
Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. 2011;13:263–78.
Ehlert U. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology. 2013;38:1850–7.
Acknowledgements
The study was supported in part by the Herman-Danna Foundation, the Milgrome Family Foundation, and the NATO SPS Program. The authors wish to thank Amalia Tabib and Daniel Neiman for their technical assistance and advice with the methylation analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalla, C., Goltser-Dubner, T., Pevzner, D. et al. Resting mononuclear cell NR3C1 and SKA2 expression levels predict blunted cortisol reactivity to combat training stress among elite army cadets exposed to childhood adversity. Mol Psychiatry 26, 6680–6687 (2021). https://doi.org/10.1038/s41380-021-01107-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01107-z
This article is cited by
-
Decreased mononuclear cell NR3C1 SKA2 and FKPB5 expression levels among adult survivors of suicide bombing terror attacks in childhood are associated with the development of PTSD
Molecular Psychiatry (2023)
-
The role of glucocorticoid receptor gene in the association between attention deficit-hyperactivity disorder and smaller brain structures
Journal of Neural Transmission (2021)