Abstract
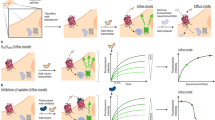
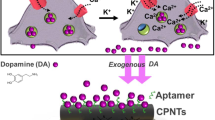
The serotonergic system in the human brain modulates several physiological processes, and altered serotonergic neurotransmission has been implicated in the neuropathology of several psychiatric disorders. The study of serotonergic neurotransmission in psychiatry has long been restricted to animal models, but advances in cell reprogramming technology have enabled the generation of serotonergic neurons from patient-induced pluripotent stem cells (iPSCs). While iPSC-derived human serotonergic neurons offer the possibility to study serotonin (5-HT) release and uptake, particularly by 5-HT-modulating drugs such as selective serotonin reuptake inhibitors (SSRIs), a major limitation is the inability to reliably quantify 5-HT secreted from neurons in vitro. Herein, we address this technical gap via a novel sensing technology that couples 5-HT-specific DNA aptamers into nanopores (glass nanopipettes) with orifices of ~10 nm to detect 5-HT in complex neuronal culture medium with higher selectivity, sensitivity, and stability than existing methods. The 5-HT aptamers undergo conformational rearrangement upon target capture and serve as gatekeepers of ionic flux through the nanopipette opening. We generated human serotonergic neurons in vitro and detected secreted 5-HT using aptamer-coated nanopipettes in a low nanomolar range, with the possibility of detecting significantly lower (picomolar) concentrations. Furthermore, as a proof of concept, we treated human serotonergic neurons in vitro with the SSRI citalopram and detected a significant increase in extracellular 5-HT using the aptamer-modified nanopipettes. We demonstrate the utility of such methods for 5-HT detection, raising the possibility of fast quantification of neurotransmitters secreted from patient-derived live neuronal cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–230.
Artigas F. Serotonin receptors involved in antidepressant effects. Pharm Ther. 2013;137:119–31.
El-Merahbi R, Löffler M, Mayer A, Sumara G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015;589:1728–34.
Vadodaria KC, Marchetto MC, Mertens J, Gage FH. Generating human serotonergic neurons in vitro: methodological advances. BioEssays. 2016;38:1123–9.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Soliman MA, Aboharb F, Zeltner N, Studer L. Pluripotent stem cells in neuropsychiatric disorders. Mol Psychiatry. 2017;22:1241–9.
Zeltner N, Studer L. Pluripotent stem cell-based disease modeling: current hurdles and future promise. Curr Opin Cell Biol. 2015;37:102–10.
Vadodaria KC, Jones JR, Linker S, Gage FH. Modeling brain disorders using induced pluripotent stem cells. Cold Spring Harb Perspect Biol. 2020;12:1–15.
Xu Z, Jiang H, Zhong P, Yan Z, Chen S, Feng J. Direct conversion of human fibroblasts to induced serotonergic neurons. Mol Psychiatry. 2016;21:62–70.
Lu J, Zhong X, Liu H, Hao L, Huang CTL, Sherafat MA, et al. Generation of serotonin neurons from human pluripotent stem cells. Nat Biotechnol. 2016;34:89–94.
Vadodaria KC, Mertens J, Paquola A, Bardy C, Li X, Jappelli R, et al. Generation of functional human serotonergic neurons from fibroblasts. Mol Psychiatry. 2016;21:49–61.
Vadodaria KC, Stern S, Marchetto MC, Gage FH. Serotonin in psychiatry: in vitro disease modeling using patient-derived neurons. Cell Tissue Res. 2018;371:161–70.
Danaceau JP, Anderson GM, McMahon WM, Crouch DJ. A liquid chromatographic-tandem mass spectrometric method for the analysis of serotonin and related indoles in human whole blood. J Anal Toxicol. 2003;27:440–4.
Johnsen E, Leknes S, Wilson SR, Lundanes E. Liquid chromatography-mass spectrometry platform for both small neurotransmitters and neuropeptides in blood, with automatic and robust solid phase extraction. Sci Rep. 2015;5:1–8.
Liu Y, Zhang J, Xu X, Zhao MK, Andrews AM, Weber SG. Capillary ultrahigh performance liquid chromatography with elevated temperature for sub-one minute separations of basal serotonin in submicroliter brain microdialysate samples. Anal Chem. 2010;82:9611–6.
Saylor RA, Hersey M, West A, Buchanan AM, Berger SN, Nijhout HF, et al. In vivo hippocampal serotonin dynamics in male and female mice: Determining effects of acute escitalopram using fast scan cyclic voltammetry. Front Neurosci. 2019;13:1–13.
Dankoski EC, Mark Wightman R. Monitoring serotonin signaling on a subsecond time scale. Front Integr Neurosci. 2013;7:1–13.
Dunham KE, Venton BJ. Improving serotonin fast-scan cyclic voltammetry detection: new waveforms to reduce electrode fouling. Analyst. 2020;145:7437–46.
Sharma S, Singh N, Tomar V, Chandra R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens Bioelectron. 2018;107:76–93.
Nichkova MI, Huisman H, Wynveen PM, Marc DT, Olson KL, Kellermann GH. Evaluation of a novel ELISA for serotonin: urinary serotonin as a potential biomarker for depression. Anal Bioanal Chem. 2012;402:1593–600.
Shaif NA, Chang DH, Cho D, Kim S, Seo DB, Shim I. The antidepressant-like effect of lactate in an animal model of menopausal depression. Biomedicines. 2018;6:1–15.
Vadodaria KC, Ji Y, Skime M, Paquola AC, Nelson T, Hall-Flavin D, et al. Altered serotonergic circuitry in SSRI-resistant major depressive disorder patient-derived neurons. Mol Psychiatry. 2019;24:808–18.
Jeong S, Yang D, Beyene AG, Del Bonis-O’Donnell JT, Gest AMM, Navarro N, et al. High-throughput evolution of near-infrared serotonin nanosensors. Sci Adv. 2019;5:1–12.
Hettie KS, Glass TE. Turn-on near-infrared fluorescent sensor for selectively imaging serotonin. ACS Chem Neurosci. 2016;7:21–5.
Ramon-Marquez T, Medina-Castillo AL, Fernandez-Gutierrez A, Fernandez-Sanchez JF. A novel optical biosensor for direct and selective determination of serotonin in serum by solid surface-room temperature phosphorescence. Biosens Bioelectron. 2016;82:217–23.
Wan J, Wanling P, Li X, Qian T, Song K, Zeng J, et al. A genetically encoded GRAB sensor for measuring serotonin dynamics in vivo. BioRxiv 2020. https://doi.org/10.1101/2020.02.24.962282.
Cui L, Lu H, Lee YH. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom Rev. 2018;37:772–92.
Baker M. Reproducibility Crisis: Blame it on the antibodies. Nature. 2015;521:274–6.
Nakatsuka N, Failletaz A, Eggemann D, Forro C, Voros J, Momotenko D. Aptamer conformational change enables serotonin biosensing with nanopipettes. Anal Chem. 2021;93:4033–41.
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–22.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–10.
Nakatsuka N, Yang K-A, Abendroth JM, Cheung KM, Xu X, Yang H, et al. Aptamer–field-effect transistors overcome Debye length limitations for small-molecule sensing. Science. 2018;362:319–24.
Vadodaria KC, Ji Y, Skime M, Paquola A, Nelson T, Hall-Flavin D, et al. Serotonin-induced hyperactivity in SSRI-resistant major depressive disorder patient-derived neurons. Mol Psychiatry. 2019;24:795–807.
Santos R, Vadodaria KC, Jaeger BN, Mei A, Lefcochilos-Fogelquist S, Mendes APD, et al. Differentiation of inflammation-responsive astrocytes from glial progenitors generated from human induced pluripotent stem cells. Stem Cell Rep. 2017;6:1757–69.
Li Y, Fleischer CM, Ross AE. High Young’s modulus carbon fibers are fouling resistant with fast-scan cyclic voltammetry. Chem Commun. 2020;56:8023–6.
Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman RM. Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal Chem. 2009;81:9462.
Pang S, Cowen S. A generic standard additions based method to determine endogenous analyte concentrations by immunoassays to overcome complex biological matrix interference. Sci Rep. 2017;7:1–10.
Aggarwal S, Mortensen OV. Overview of monoamine transporters. Curr Protoc Pharm. 2017;79:12.16.1–7.
Perez-Gonzalez C, Lafontaine DA, Penedo JC. Fluorescence-based strategies to investigate the structure and dynamics of aptamer-ligand complexes. Front Chem. 2016;4:1–22.
Kypr J, Kejnovská I, Renčiuk D, Vorlíčková M. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res. 2009;37:1713–25.
Neumann O, Zhang D, Tam F, Lal S, Wittung-Stafshede P, Halas NJ. Direct optical detection of aptamer conformational changes induced by target molecules. Anal Chem. 2009;81:10002–6.
Yang KA, Pei R, Stojanovic MN. In vitro selection and amplification protocols for isolation of aptameric sensors for small molecules. Methods. 2016;106:58–65.
Feagin TA, Maganzini N, Soh HT. Strategies for creating structure-switching aptamers. ACS Sens. 2018;3:1611–5.
Oh SS, Plakos K, Lou X, Xiao Y, Soh HT. In vitro selection of structure-switching, self-reporting aptamers. Proc Natl Acad Sci USA. 2010;107:14053–8.
Nutiu R, Li Y. Structure-switching signaling aptamers. J Am Chem Soc. 2003;125:4771–8.
Yang K-A, Barbu M, Halim M, Pallavi P, Kim B, Kolpashchikov DM, et al. Recognition and sensing of low-epitope targets via ternary complexes with oligonucleotides and synthetic receptors. Nat Chem. 2014;6:1003–8.
Actis P, Mak AC, Pourmand N. Functionalized nanopipettes: toward label-free, single cell biosensors. Bioanal Rev. 2010;1:177–85.
Deamer D, Akeson M, Branton D. Three decades of nanopore sequencing. Nat Biotechnol. 2016;34:518–24.
Venkatesan BM, Bashir R. Nanopore sensors for nucleic acid analysis. Nat Nanotechnol. 2011;6:615–24.
Yao F, Peng X, Su Z, Tian L, Guo Y, Kang XF. Crowding-induced DNA translocation through a protein nanopore. Anal Chem. 2020;92:3827–33.
Chau CC, Radford SE, Hewitt EW, Actis P. Macromolecular crowding enhances the detection of DNA and proteins by a solid-state nanopore. Nano Lett. 2020;20:5553–61.
Kishida KT, Sandberg SG, Lohrenz T, Comair YG, Sáez I, Phillips PEM, et al. Sub-second dopamine detection in human striatum. PLoS ONE. 2011;6:e23291.
Seaton BT, Hill DF, Cowen SL, Heien ML. Mitigating the effects of electrode biofouling-induced impedance for improved long-term electrochemical measurements in vivo. Anal Chem. 2020;92:6334–40.
Wrona MZ, Dryhurst G. Electrochemical oxidation of 5-hydroxytryptamine in aqueous solution at physiological pH. Bioorg Chem. 1990;18:291–317.
Puthongkham P, Lee ST, Jill Venton B. Mechanism of histamine oxidation and electropolymerization at carbon electrodes. Anal Chem. 2019;91:8366–73.
Jackson BP, Dietz SM, Wightman RM. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal Chem. 1995;67:1115–20.
Eggenberger OM, Ying C, Mayer M. Surface coatings for solid-state nanopores. Nanoscale. 2019;11:19636–57.
Umehara S, Karhanek M, Davis RW, Pourmand N. Label-free biosensing with functionalized nanopipette probes. Proc Natl Acad Sci USA. 2009;106:4611–6.
Abelow AE, Schepelina O, White RJ, Vallée-Bélisle A, Plaxco KW, Zharov I. Biomimetic glass nanopores employing aptamer gates responsive to a small molecule. Chem Commun. 2010;42:7984–6.
Actis P, Rogers A, Nivala J, Vilozny B, Seger RA, Jejelowo O, et al. Reversible thrombin detection by aptamer functionalized STING sensors. Biosens Bioelectron. 2011;26:4503–7.
Cai SL, Cao SH, Zheng YB, Zhao S, Yang JL, Li YQ. Surface charge modulated aptasensor in a single glass conical nanopore. Biosens Bioelectron. 2015;71:37–43.
Taylor PJ. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin Biochem. 2005;38:328–34.
Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30.
Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–515.
Kantner T, Watts AG. Characterization of reactions between water-soluble trialkylphosphines and thiol alkylating reagents: implications for protein-conjugation reactions. Bioconjug Chem. 2016;27:2400–6.
Acknowledgements
This research was supported by the Takeda-Sanford Consortium Innovation Alliance grant program and the JPB foundation. This project received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement number GrapheneCore3 881603. KCV was supported by the Swiss National Science Foundation (SNSF) outgoing postdoctoral fellowship. Research supported in part by the American Heart Association and the Paul G. Allen Frontiers Group Grant #19PABHI34610000/TEAM LEADER: Fred H. Gage/2019, Annette C. Merle-Smith, Robert and Mary Jane Engman Foundation, and Lynn and Edward Streim. NN was supported by the ETH Zurich Postdoctoral Fellowship (COFUND 18-1 FEL-35). DM was supported by the Swiss National Science Foundation Ambizione Grant (PZ00P2_174217/1). The authors acknowledge Prof. Unwin for sharing the WEC-SPM software package that was used to control the nanopipette instrument and Dr. Alex Colburn for supplying a low-noise current amplifier (University of Warwick). The authors also acknowledge technical assistance from Stephen Wheeler (ETH Zurich).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nakatsuka, N., Heard, K.J., Faillétaz, A. et al. Sensing serotonin secreted from human serotonergic neurons using aptamer-modified nanopipettes. Mol Psychiatry 26, 2753–2763 (2021). https://doi.org/10.1038/s41380-021-01066-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01066-5