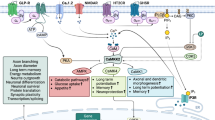
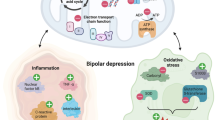
Abstract
Given its chronicity, contribution to disability and morbidity, and prevalence of more than 2%, the effective treatment, and prevention of bipolar disorder represents an area of significant unmet medical need. While more than half a century has passed since the introduction of lithium into widespread use at the birth of modern psychopharmacology, that medication remains a mainstay for the acute treatment and prevention of recurrent mania/hypomania and depression that characterize bipolar disorder. However, the continued limited understanding of how lithium modulates affective behavior and lack of validated cellular and animal models have resulted in obstacles to discovering more effective mood stabilizers with fewer adverse side effects. In particular, while there has been progress in developing new pharmacotherapy for mania, developing effective treatments for acute bipolar depression remain inadequate. Recent large-scale human genetic studies have confirmed the complex, polygenic nature of the risk architecture of bipolar disorder, and its overlap with other major neuropsychiatric disorders. Such discoveries have begun to shed light on the pathophysiology of bipolar disorder. Coupled with broader advances in human neurobiology, neuropharmacology, noninvasive neuromodulation, and clinical trial design, we can envision novel therapeutic strategies informed by defined molecular mechanisms and neural circuits and targeted to the root cause of the pathophysiology. Here, we review recent advances toward the goal of better treatments for bipolar disorder, and we outline major challenges for the field of translational neuroscience that necessitate continued focus on fundamental research and discovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cade JF. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2:349–52.
https://enacademic.com/dic.nsf/enwiki/9778 Accessed 1 July 2020.
Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–52.
Schioldann J. History of the introduction of lithium into medicine and psychiatry: birth of modern psychopharmacology. Adelaide: Academic Press; 1949.
Parker G, McCraw S, Hadzi-Pavlovic D, Fletcher K. Costs of the principal mood disorders: a study of comparative direct and indirect costs incurred by those with bipolar I, bipolar II and unipolar disorders. J Affect Disord. 2013;149:46–55.
Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60:261–9.
Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–7.
Tohen M, Hennen J, Zarate CM Jr, Baldessarini RJ, Strakowski SM, Stoll AL, et al. Two-year syndromal and functional recovery in 219 cases of first-episode major affective disorder with psychotic features. Am J Psychiatry. 2000;157:220–8.
Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–50.
Keck PE, Perlis RH, Otto MW, Carpenter D, Ross R, Docherty JP. Expert Consensus Guideline Series: Treatment of Bipolar Disorder 2004. Postgrad Med. 2004:1–120.
Hirschfeld RA, Bowden CL, Gitlin MJ, Keck PE, Perlis RH, Suppes T et al. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(Suppl 4):1–11.
Suppes T, Dennehy EB, Hirschfeld RM, Altshuler LL, Bowden CL, Calabrese JR, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66:870–86.
Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–22.
Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, et al. Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry. 2001;158:906–12.
Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord. 2008;10:323–33.
Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60:1079–88.
Thase ME, Macfadden W, Weisler RH, Chang W, Paulsson B, Khan A, et al. Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study). J Clin Psychopharmacol. 2006;26:600–9.
Vieta E, Calabrese JR, Goikolea JM, Raines S, Macfadden W. Quetiapine monotherapy in the treatment of patients with bipolar I or II depression and a rapid-cycling disease course: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2007;9:413–25.
Weisler RH, Calabrese JR, Thase ME, Arvekvist R, Stening G, Paulsson B, et al. Efficacy of quetiapine monotherapy for the treatment of depressive episodes in bipolar I disorder: a post hoc analysis of combined results from 2 double-blind, randomized, placebo-controlled studies. J Clin Psychiatry. 2008;69:769–82.
Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19:1–93.
Scherk H, Pajonk FG, Leucht S. Second-generation antipsychotic agents in the treatment of acute mania: a systematic review and meta-analysis of randomized controlled trials. Arch Gen Psychiatry. 2007;64:442–55.
Cosgrove VE, Kelsoe JR, Suppes T. Toward a valid animal model of bipolar disorder: how the research domain criteria help bridge the clinical-basic science divide. Biol Psychiatry. 2016;79:62–70.
Scolnick EM. The path to new therapies for schizophrenia and bipolar illness. FASEB J. 2017;31:1254–9.
Chen HM, DeLong CJ, Bame M, Rajapakse I, Herron TJ, McInnis MG, et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl Psychiatry. 2014;4:e375.
Wang JL, Shamah SM, Sun AX, Waldman ID, Haggarty SJ, Perlis RH. Label-free, live optical imaging of reprogrammed bipolar disorder patient-derived cells reveals a functional correlate of lithium responsiveness. Transl Psychiatry. 2014;4:e428.
Madison JM, Zhou F, Nigam A, Hussain A, Barker DD, Nehme R, et al. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol Psychiatry. 2015;20:703–17.
Bavamian S, Mellios N, Lalonde J, Fass DM, Wang J, Sheridan SD, et al. Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol Psychiatry. 2015;20:573–84.
Mertens J, Wang QW, Kim Y, Yu DX, Pham S, Yang B, et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527:95–99.
Kim KH, Liu J, Sells Galvin RJ, Dage JL, Egeland JA, Smith RC, et al. Transcriptomic analysis of induced pluripotent stem cells derived from patients with bipolar disorder from an old order amish pedigree. PLoS ONE. 2015;10:e0142693.
Tobe BTD, Crain AM, Winquist AM, Calabrese B, Makihara H, Zhao WN, et al. Probing the lithium-response pathway in hiPSCs implicates the phosphoregulatory set-point for a cytoskeletal modulator in bipolar pathogenesis. Proc Natl Acad Sci USA. 2017;114:E4462–71.
Vizlin-Hodzic D, Zhai Q, Illes S, Sodersten K, Truve K, Parris TZ, et al. Early onset of inflammation during ontogeny of bipolar disorder: the NLRP2 inflammasome gene distinctly differentiates between patients and healthy controls in the transition between iPS cell and neural stem cell stages. Transl Psychiatry. 2017;7:e1010.
Stern S, Santos R, Marchetto MC, Mendes APD, Rouleau GA, Biesmans S, et al. Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Mol Psychiatry. 2018;23:1453–65.
Haggarty SJ, Silva MC, Cross A, Brandon NJ, Perlis RH. Advancing drug discovery for neuropsychiatric disorders using patient-specific stem cell models. Mol Cell Neurosci. 2016;73:104–15.
Nehme R, Zuccaro E, Ghosh SD, Li C, Sherwood JL, Pietilainen O, et al. Combining NGN2 programming with developmental patterning generates human excitatory neurons with NMDAR-mediated synaptic transmission. Cell Rep. 2018;23:2509–23.
Marton RM, Pasca SP. Organoid and assembloid technologies for investigating cellular crosstalk in human brain development and disease. Trends Cell Biol. 2019;30:133–43.
Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 2019;570:523–7.
NIMH Repository & Genomics Resource (https://www.nimhgenetics.org). 2020.
Insel TR. The NIMH research domain criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–7.
McCoy TH Jr, Castro VM, Hart KL, Pellegrini AM, Yu S, Cai T, et al. Genome-wide association study of dimensional psychopathology using electronic health records. Biol Psychiatry. 2018;83:1005–11.
Lago SG, Tomasik J, van Rees GF, Steeb H, Cox DA, Rustogi N, et al. Drug discovery for psychiatric disorders using high-content single-cell screening of signaling network responses ex vivo. Sci Adv. 2019;5:eaau9093.
Perlis RH. Translating biomarkers to clinical practice. Mol Psychiatry. 2011;16:1076–87.
Rueckert EH, Barker D, Ruderfer D, Bergen SE, O’Dushlaine C, Luce CJ, et al. Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Mol Psychiatry. 2013;18:922–9.
Hughes T, Hansson L, Sonderby IE, Athanasiu L, Zuber V, Tesli M, et al. A loss-of-function variant in a minor isoform of ANK3 protects against bipolar disorder and schizophrenia. Biol Psychiatry. 2016;80:323–30.
Hughes T, Sonderby IE, Polushina T, Hansson L, Holmgren A, Athanasiu L, et al. Elevated expression of a minor isoform of ANK3 is a risk factor for bipolar disorder. Transl Psychiatry. 2018;8:210.
Leussis MP, Berry-Scott EM, Saito M, Jhuang H, de Haan G, Alkan O, et al. The ANK3 bipolar disorder gene regulates psychiatric-related behaviors that are modulated by lithium and stress. Biol Psychiatry. 2013;73:683–90.
Gottschalk MG, Leussis MP, Ruland T, Gjeluci K, Petryshen TL, Bahn S. Lithium reverses behavioral and axonal transport-related changes associated with ANK3 bipolar disorder gene disruption. Eur Neuropsychopharmacol. 2017;27:274–88.
Zhu S, Cordner ZA, Xiong J, Chiu CT, Artola A, Zuo Y, et al. Genetic disruption of ankyrin-G in adult mouse forebrain causes cortical synapse alteration and behavior reminiscent of bipolar disorder. Proc Natl Acad Sci USA. 2017;114:10479–84.
van der Werf IM, Van Dam D, Missault S, Yalcin B, De Deyn PP, Vandeweyer G, et al. Behavioural characterization of AnkyrinG deficient mice, a model for ANK3 related disorders. Behav Brain Res. 2017;328:218–26.
Lopez AY, Wang X, Xu M, Maheshwari A, Curry D, Lam S, et al. Ankyrin-G isoform imbalance and interneuronopathy link epilepsy and bipolar disorder. Mol Psychiatry. 2017;22:1464–72.
Nelson AD, Caballero-Floran RN, Rodriguez Diaz JC, Hull JM, Yuan Y, Li J et al. Ankyrin-G regulates forebrain connectivity and network synchronization via interaction with GABARAP. Mol Psychiatry. 2018. https://doi.org/10.1038/s41380-018-0308-x.
Garza JC, Qi X, Gjeluci K, Leussis MP, Basu H, Reis SA, et al. Disruption of the psychiatric risk gene Ankyrin 3 enhances microtubule dynamics through GSK3/CRMP2 signaling. Transl Psychiatry. 2018;8:135.
Ellis LD, Soanes KH. A larval zebrafish model of bipolar disorder as a screening platform for neuro-therapeutics. Behav Brain Res. 2012;233:450–7.
Lin X, Duan X, Jacobs C, Ullmann J, Chan CY, Chen S, et al. High-throughput brain activity mapping and machine learning as a foundation for systems neuropharmacology. Nat Commun. 2018;9:5142.
Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–9.
Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–8.
Freland L, Beaulieu JM. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front Mol Neurosci. 2012;5:14.
Ryves WJ, Dajani R, Pearl L, Harwood AJ. Glycogen synthase kinase-3 inhibition by lithium and beryllium suggests the presence of two magnesium binding sites. Biochem Biophys Res Commun. 2002;290:967–72.
Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296:15–19.
Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci USA. 1999;96:8745–50.
De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–64.
Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–77.
Li X, Friedman AB, Zhu W, Wang L, Boswell S, May RS, et al. Lithium regulates glycogen synthase kinase-3beta in human peripheral blood mononuclear cells: implication in the treatment of bipolar disorder. Biol Psychiatry. 2007;61:216–22.
Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–74.
Haggarty SJ, Singh K, Perlis RH, Karmacharya R. Wnt signaling in mood & psychotic disorders. In: Wnt signaling in development and disease: molecular mechanisms and biological functions. Vol. 29. USA: Wiley; 2014. pp. 379–392.
Haggarty SJ, Singh K, Perlis RH, Karmacharya R. Neuropsychiatric disease-associated genetic variation in the Wnt pathway. In: Wnt signaling in development and disease: molecular mechanisms and biological functions. vol. 30. USA: Wiley; 2014. pp. 393–410.
Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–104.
Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29:32–38.
O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–8.
Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–37.
Gould TD, Einat H, O’Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–83.
Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–36.
Pan JQ, Lewis MC, Ketterman JK, Clore EL, Riley M, Richards KR, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology. 2011;36:1397–11.
Harris TJ, Peifer M. Decisions, decisions: beta-catenin chooses between adhesion and transcription. Trends Cell Biol. 2005;15:234–7.
Gould TD, Manji HK. The Wnt signaling pathway in bipolar disorder. Neuroscientist. 2002;8:497–511.
Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry. 2008;13:285–92.
Zhao WN, Cheng C, Theriault KM, Sheridan SD, Tsai LH, Haggarty SJ. A high-throughput screen for Wnt/beta-catenin signaling pathway modulators in human iPSC-derived neural progenitors. J Biomol Screen. 2012;17:1252–63.
Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–26.
Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86:281–96.
Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–34.
Choe Y, Pleasure SJ. Wnt signaling regulates intermediate precursor production in the postnatal dentate gyrus by regulating CXCR4 expression. Dev Neurosci. 2012;34:502–14.
Snitow ME, Zanni G, Ciesielski B, Burgess-Jones P, Eisch AJ, O’Brien WT, et al. Adult hippocampal neurogenesis is not necessary for the response to lithium in the forced swim test. Neurosci Lett. 2019;704:67–72.
Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–31.
Lipina TV, Kaidanovich-Beilin O, Patel S, Wang M, Clapcote SJ, Liu F, et al. Genetic and pharmacological evidence for schizophrenia-related Disc1 interaction with GSK-3. Synapse. 2011;65:234–48.
Hennig KM, Fass DM, Zhao WN, Sheridan SD, Fu T, Erdin S, et al. WNT/beta-catenin pathway and epigenetic mechanisms regulate the Pitt-Hopkins syndrome and schizophrenia risk gene TCF4. Mol Neuropsychiatry. 2017;3:53–71.
Kuechler A, Willemsen MH, Albrecht B, Bacino CA, Bartholomew DW, van Bokhoven H, et al. De novo mutations in beta-catenin (CTNNB1) appear to be a frequent cause of intellectual disability: expanding the mutational and clinical spectrum. Hum Genet. 2015;134:97–109.
Krumm N, O’Roak BJ, Shendure J, Eichler EE. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 2014;37:95–105.
Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73.
O’Brien WT, Huang J, Buccafusca R, Garskof J, Valvezan AJ, Berry GT, et al. Glycogen synthase kinase-3 is essential for beta-arrestin-2 complex formation and lithium-sensitive behaviors in mice. J Clin Invest. 2011;121:3756–62.
Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3beta in D2R-expressing neurons reveals distinct roles for beta-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci USA. 2012;109:20732–7.
Latapy C, Rioux V, Guitton MJ, Beaulieu JM. Selective deletion of forebrain glycogen synthase kinase 3beta reveals a central role in serotonin-sensitive anxiety and social behaviour. Philos Trans R Soc Lond B Biol Sci. 2012;367:2460–74.
Kim W, Won SY, Yoon BJ. CRMP2 mediates GSK3beta actions in the striatum on regulating neuronal structure and mania-like behavior. J Affect Disord. 2019;245:1079–88.
Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, et al. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA. 2008;105:13656–61.
Khlghatyan J, Evstratova A, Chamberland S, Marakhovskaia A, Bahremand A, Toth K, et al. Mental Illnesses-Associated Fxr1 and Its Negative Regulator Gsk3beta Are Modulators of Anxiety and Glutamatergic Neurotransmission. Front Mol Neurosci. 2018;11:119.
Del’Guidice T, Latapy C, Rampino A, Khlghatyan J, Lemasson M, Gelao B, et al. FXR1P is a GSK3beta substrate regulating mood and emotion processing. Proc Natl Acad Sci USA. 2015;112:E4610–9.
Li YC, Panikker P, Xing B, Yang SS, Alexandropoulos C, McEachern EP, et al. Deletion of glycogen synthase kinase-3beta in D2 receptor-positive neurons ameliorates cognitive impairment via nmda receptor-dependent synaptic plasticity. Biol Psychiatry. 2020;87:745–55.
Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–41.
Fass DM, Shah R, Ghosh B, Hennig K, Norton S, Zhao WN, et al. Effect of inhibiting histone deacetylase with short-chain carboxylic acids and their hydroxamic acid analogs on vertebrate development and neuronal chromatin. ACS Med Chem Lett. 2010;2:39–42.
Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64.
Yamawaki Y, Fuchikami M, Morinobu S, Segawa M, Matsumoto T, Yamawaki S. Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus. World J Biol Psychiatry. 2012;13:458–67.
Arent CO, Valvassori SS, Fries GR, Stertz L, Ferreira CL, Lopes-Borges J, et al. Neuroanatomical profile of antimaniac effects of histone deacetylases inhibitors. Mol Neurobiol. 2011;43:207–14.
Kim WY, Kim S, Kim JH. Chronic microinjection of valproic acid into the nucleus accumbens attenuates amphetamine-induced locomotor activity. Neurosci Lett. 2008;432:54–57.
Covington HE III, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–60.
Covington HE III, Vialou VF, LaPlant Q, Ohnishi YN, Nestler EJ. Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci Lett. 2011;493:122–6.
Schroeder FA, Lewis MC, Fass DM, Wagner FF, Zhang YL, Hennig KM, et al. A selective HDAC 1/2 inhibitor modulates chromatin and gene expression in brain and alters mouse behavior in two mood-related tests. PLoS ONE. 2013;8:e71323.
Covington HE III, Maze I, Vialou V, Nestler EJ. Antidepressant action of HDAC inhibition in the prefrontal cortex. Neuroscience. 2015;298:329–35.
Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60.
Wagner FF, Zhang YL, Fass DM, Joseph N, Gale JP, Weiwer M, et al. Kinetically selective inhibitors of histone deacetylase 2 (HDAC2) as cognition enhancers. Chem Sci. 2015;6:804–15.
An WF, Germain AR, Bishop JA, Nag PP, Metkar S, Ketterman J, et al. Discovery of Potent and Highly Selective Inhibitors of GSK3b. Bethesda (MD): Probe Reports from the NIH Molecular Libraries Program; 2010.
Georgievska B, Sandin J, Doherty J, Mortberg A, Neelissen J, Andersson A, et al. AZD1080, a novel GSK3 inhibitor, rescues synaptic plasticity deficits in rodent brain and exhibits peripheral target engagement in humans. J Neurochem. 2013;125:446–56.
Wagner FF, Benajiba L, Campbell AJ, Weiwer M, Sacher JR, Gale JP et al. Exploiting an Asp-Glu “switch” in glycogen synthase kinase 3 to design paralog-selective inhibitors for use in acute myeloid leukemia. Sci Transl Med. 2018;10:eaam8460.
Wagner FF, Bishop JA, Gale JP, Shi X, Walk M, Ketterman J, et al. Inhibitors of glycogen synthase kinase 3 with exquisite kinome-wide selectivity and their functional effects. ACS Chem Biol. 2016;11:1952–63.
Griebel G, Stemmelin J, Lopez-Grancha M, Boulay D, Boquet G, Slowinski F, et al. The selective GSK3 inhibitor, SAR502250, displays neuroprotective activity and attenuates behavioral impairments in models of neuropsychiatric symptoms of Alzheimer’s disease in rodents. Sci Rep. 2019;9:18045.
Bernard-Gauthier V, Mossine AV, Knight A, Patnaik D, Zhao WN, Cheng C, et al. Structural basis for achieving GSK-3beta inhibition with high potency, selectivity, and brain exposure for positron emission tomography imaging and drug discovery. J Med Chem. 2019;62:9600–17.
Bhat RV, Andersson U, Andersson S, Knerr L, Bauer U, Sundgren-Andersson AK. The conundrum of gsk3 inhibitors: is it the dawn of a new beginning? J Alzheimers Dis. 2018;64:S547–54.
Zhao WN. Discovery of suppressors of CRMP2 phosphorylation reveals compounds that mimic the behavioral effects of lithium on amphetamine-induced hyperlocomotion. Transl Psychiatry. 2020;10:76.
Soutar MP, Kim WY, Williamson R, Peggie M, Hastie CJ, McLauchlan H, et al. Evidence that glycogen synthase kinase-3 isoforms have distinct substrate preference in the brain. J Neurochem. 2010;115:974–83.
Caberlotto L, Carboni L, Zanderigo F, Andreetta F, Andreoli M, Gentile G, et al. Differential effects of glycogen synthase kinase 3 (GSK3) inhibition by lithium or selective inhibitors in the central nervous system. Naunyn Schmiedebergs Arch Pharm. 2013;386:893–903.
Bocchetta A, Bernardi F, Burrai C, Pedditzi M, Del Zompo M. A double-blind study of L-sulpiride versus amitriptyline in lithium-maintained bipolar depressives. Acta Psychiatr Scand. 1993;88:434–9.
Zheng W, Xiang YQ, Li XB, Ungvari GS, Chiu HF, Sun F, et al. Adjunctive huperzine A for cognitive deficits in schizophrenia: a systematic review and meta-analysis. Hum Psychopharmacol. 2016;31:286–95.
Ratia M, Gimenez-Llort L, Camps P, Munoz-Torrero D, Perez B, Clos MV, et al. Huprine X and huperzine A improve cognition and regulate some neurochemical processes related with Alzheimer’s disease in triple transgenic mice (3xTg-AD). Neurodegener Dis. 2013;11:129–40.
Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–9.
Atack JR, Broughton HB, Pollack SJ. Inositol monophosphatase–a putative target for Li+ in the treatment of bipolar disorder. Trends Neurosci. 1995;18:343–9.
Singh N, Halliday AC, Thomas JM, Kuznetsova OV, Baldwin R, Woon EC, et al. A safe lithium mimetic for bipolar disorder. Nat Commun. 2013;4:1332.
Barkus C, Ferland JN, Adams WK, Churchill GC, Cowen PJ, Bannerman DM, et al. The putative lithium-mimetic ebselen reduces impulsivity in rodent models. J Psychopharmacol. 2018;32:1018–26.
Tondo L, Baldessarini RJ. Antisuicidal effects in mood disorders: are they unique to lithium? Pharmacopsychiatry. 2018;51:177–88.
Singh N, Sharpley AL, Emir UE, Masaki C, Herzallah MM, Gluck MA, et al. Effect of the putative lithium mimetic ebselen on brain myo-inositol, sleep, and emotional processing in humans. Neuropsychopharmacology. 2016;41:1768–78.
Masaki C, Sharpley AL, Cooper CM, Godlewska BR, Singh N, Vasudevan SR, et al. Effects of the potential lithium-mimetic, ebselen, on impulsivity and emotional processing. Psychopharmacol (Berl). 2016;233:2655–61.
Craddock N, Sklar P. Genetics of bipolar disorder. Lancet. 2013;381:1654–62.
Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803.
Cross-Disorder Group of the Psychiatric Genomics Consortium. Electronic address pmhe, Cross-Disorder Group of the Psychiatric Genomics C. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179:1469–82. e1411.
Hoffman GE, Bendl J, Voloudakis G, Montgomery KS, Sloofman L, Wang YC, et al. CommonMind Consortium provides transcriptomic and epigenomic data for schizophrenia and bipolar disorder. Sci Data. 2019;6:180.
Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Disco. 2013;12:581–94.
Cipriani A, Saunders K, Attenburrow MJ, Stefaniak J, Panchal P, Stockton S, et al. A systematic review of calcium channel antagonists in bipolar disorder and some considerations for their future development. Mol Psychiatry. 2016;21:1324–32.
Kabir ZD, Martinez-Rivera A, Rajadhyaksha AM. From gene to behavior: L-type calcium channel mechanisms underlying neuropsychiatric symptoms. Neurotherapeutics. 2017;14:588–613.
Brunet G, Cerlich B, Robert P, Dumas S, Souetre E, Darcourt G. Open trial of a calcium antagonist, nimodipine, in acute mania. Clin Neuropharmacol. 1990;13:224–8.
Grunze H, Walden J, Wolf R, Berger M. Combined treatment with lithium and nimodipine in a bipolar I manic syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:419–26.
Pazzaglia PJ, Post RM, Ketter TA, George MS, Marangell LB. Preliminary controlled trial of nimodipine in ultra-rapid cycling affective dysregulation. Psychiatry Res. 1993;49:257–72.
Pazzaglia PJ, Post RM, Ketter TA, Callahan AM, Marangell LB, Frye MA, et al. Nimodipine monotherapy and carbamazepine augmentation in patients with refractory recurrent affective illness. J Clin Psychopharmacol. 1998;18:404–13.
Ostacher MJ, Iosifescu DV, Hay A, Blumenthal SR, Sklar P, Perlis RH. Pilot investigation of isradipine in the treatment of bipolar depression motivated by genome-wide association. Bipolar Disord. 2014;16:199–203.
Atkinson LZ, Colbourne L, Smith A, Harmer CH, Nobre AC, Rendell J, et al. The oxford study of calcium channel antagonism, cognition, mood instability and sleep (OxCaMS): study protocol for a randomised controlled, experimental medicine study. Trials. 2019;20:120.
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25.
Renthal W, Maze I, Krishnan V, Covington HE III, Xiao G, Kumar A, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–29.
Wey HY, Gilbert TM, Zurcher NR, She A, Bhanot A, Taillon BD, et al. Insights into neuroepigenetics through human histone deacetylase PET imaging. Sci Transl Med. 2016;8:351ra106.
Gilbert TM, Zurcher NR, Wu CJ, Bhanot A, Hightower BG, Kim M, et al. PET neuroimaging reveals histone deacetylase dysregulation in schizophrenia. J Clin Invest. 2019;129:364–72.
Arjmand S, Behzadi M, Kohlmeier KA, Mazhari S, Sabahi A, Shabani M. Bipolar disorder and the endocannabinoid system. Acta Neuropsychiatr. 2019;31:193–201.
Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29.
McIntyre RS, Soczynska JK, Woldeyohannes HO, Miranda A, Vaccarino A, Macqueen G, et al. A randomized, double-blind, controlled trial evaluating the effect of intranasal insulin on neurocognitive function in euthymic patients with bipolar disorder. Bipolar Disord. 2012;14:697–706.
Loebel A, Xu J, Hsu J, Cucchiaro J, Pikalov A. The development of lurasidone for bipolar depression. Ann N Y Acad Sci. 2015;1358:95–104.
DelBello MP, Goldman R, Phillips D, Deng L, Cucchiaro J, Loebel A. Efficacy and safety of lurasidone in children and adolescents with bipolar I depression: a double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 2017;56:1015–25.
Ishiyama T, Tokuda K, Ishibashi T, Ito A, Toma S, Ohno Y. Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur J Pharm. 2007;572:160–70.
Yatham LN, Mackala S, Basivireddy J, Ahn S, Walji N, Hu C, et al. Lurasidone versus treatment as usual for cognitive impairment in euthymic patients with bipolar I disorder: a randomised, open-label, pilot study. Lancet Psychiatry. 2017;4:208–17.
Harvey PD, Ogasa M, Cucchiaro J, Loebel A, Keefe RS. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. ziprasidone. Schizophr Res. 2011;127:188–94.
Enomoto T, Ishibashi T, Tokuda K, Ishiyama T, Toma S, Ito A. Lurasidone reverses MK-801-induced impairment of learning and memory in the Morris water maze and radial-arm maze tests in rats. Behav Brain Res. 2008;186:197–207.
Horisawa T, Ishibashi T, Nishikawa H, Enomoto T, Toma S, Ishiyama T, et al. The effects of selective antagonists of serotonin 5-HT7 and 5-HT1A receptors on MK-801-induced impairment of learning and memory in the passive avoidance and Morris water maze tests in rats: mechanistic implications for the beneficial effects of the novel atypical antipsychotic lurasidone. Behav Brain Res. 2011;220:83–90.
Van Rheenen TE, Lewandowski KE, Bauer IE, Kapczinski F, Miskowiak K, Burdick KE et al. Current understandings of the trajectory and emerging correlates of cognitive impairment in bipolar disorder: An overview of evidence. Bipolar Disord. 2019;22:13–27.
Cuomo I, Piacentino D, Kotzalidis GD, Lionetto L, De Filippis S. Lacosamide in bipolar disorder: A 30-day comparison to a retrospective control group treated with other antiepileptics. Psychiatry Clin Neurosci. 2018;72:864–75.
Wilson SM, Khanna R. Specific binding of lacosamide to collapsin response mediator protein 2 (CRMP2) and direct impairment of its canonical function: implications for the therapeutic potential of lacosamide. Mol Neurobiol. 2015;51:599–609.
Fava M. The possible antianxiety and mood-stabilizing effects of rufinamide. Psychother Psychosom. 2010;79:194–5.
Mazza M, Marano G, Janiri L. Retigabine-ezogabine: a new treatment option for bipolar disorder? Bipolar Disord. 2019;21:283–4.
Amann B, Sterr A, Vieta E, Stampfer R, Walden J, Grunze H. An exploratory open trial on safety and efficacy of the anticonvulsant retigabine in acute manic patients. J Clin Psychopharmacol. 2006;26:534–6.
Dencker D, Dias R, Pedersen ML, Husum H. Effect of the new antiepileptic drug retigabine in a rodent model of mania. Epilepsy Behav. 2008;12:49–53.
Dencker D, Husum H. Antimanic efficacy of retigabine in a proposed mouse model of bipolar disorder. Behav Brain Res. 2010;207:78–83.
Kristensen LV, Sandager-Nielsen K, Hansen HH. K(v) 7 (KCNQ) channel openers normalize central 2-deoxyglucose uptake in a mouse model of mania and increase prefrontal cortical and hippocampal serine-9 phosphorylation levels of GSK3beta. J Neurochem. 2012;121:373–82.
Dedic N, Jones PG, Hopkins SC, Lew R, Shao L, Campbell JE, et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Pharm Exp Ther. 2019;371:1–14.
Harmeier A, Obermueller S, Meyer CA, Revel FG, Buchy D, Chaboz S, et al. Trace amine-associated receptor 1 activation silences GSK3beta signaling of TAAR1 and D2R heteromers. Eur Neuropsychopharmacol. 2015;25:2049–61.
Karmacharya R, Lynn SK, Demarco S, Ortiz A, Wang X, Lundy MY, et al. Behavioral effects of clozapine: involvement of trace amine pathways in C. elegans and M. musculus. Brain Res. 2011;1393:91–99.
McNamara RK, Welge JA. Meta-analysis of erythrocyte polyunsaturated fatty acid biostatus in bipolar disorder. Bipolar Disord. 2016;18:300–6.
Saunders EF, Ramsden CE, Sherazy MS, Gelenberg AJ, Davis JM, Rapoport SI. Omega-3 and omega-6 polyunsaturated fatty acids in bipolar disorder: a review of biomarker and treatment studies. J Clin Psychiatry. 2016;77:e1301–8.
Grayson DS, Kroenke CD, Neuringer M, Fair DA. Dietary omega-3 fatty acids modulate large-scale systems organization in the rhesus macaque brain. J Neurosci. 2014;34:2065–74.
Zhao WN, Hylton NK, Wang J, Chindavong PS, Alural B, Kurtser I, et al. Activation of WNT and CREB signaling pathways in human neuronal cells in response to the Omega-3 fatty acid docosahexaenoic acid (DHA). Mol Cell Neurosci. 2019;99:103386.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl J Med. 2019;380:11–22.
Nierenberg AA, Ghaznavi SA, Sande Mathias I, Ellard KK, Janos JA, Sylvia LG. Peroxisome proliferator-activated receptor gamma coactivator-1 alpha as a novel target for bipolar disorder and other neuropsychiatric disorders. Biol Psychiatry. 2018;83:761–9.
Kemp DE, Schinagle M, Gao K, Conroy C, Ganocy SJ, Ismail-Beigi F, et al. PPAR-gamma agonism as a modulator of mood: proof-of-concept for pioglitazone in bipolar depression. CNS Drugs. 2014;28:571–81.
Zeinoddini A, Sorayani M, Hassanzadeh E, Arbabi M, Farokhnia M, Salimi S, et al. Pioglitazone adjunctive therapy for depressive episode of bipolar disorder: a randomized, double-blind, placebo-controlled trial. Depress Anxiety. 2015;32:167–73.
Nutt D. Psychedelic drugs-a new era inpsychiatry? Dialogues Clin Neurosci. 2019;21:139–47.
Carhart-Harris RL, Roseman L, Bolstridge M, Demetriou L, Pannekoek JN, Wall MB, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep. 2017;7:13187.
Carhart-Harris RL, Bolstridge M, Day CMJ, Rucker J, Watts R, Erritzoe DE, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacol (Berl). 2018;235:399–408.
Madsen MK, Fisher PM, Burmester D, Dyssegaard A, Stenbaek DS, Kristiansen S, et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology. 2019;44:1328–34.
Chouinard G, Young SN, Annable L. A controlled clinical trial of L-tryptophan in acute mania. Biol Psychiatry. 1985;20:546–57.
Lindseth G, Helland B, Caspers J. The effects of dietary tryptophan on affective disorders. Arch Psychiatr Nurs. 2015;29:102–7.
Ritter PS, Marx C, Bauer M, Leopold K, Pfennig A. The role of disturbed sleep in the early recognition of bipolar disorder: a systematic review. Bipolar Disord. 2011;13:227–37.
Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165:830–43.
Seleem MA, Merranko JA, Goldstein TR, Goldstein BI, Axelson DA, Brent DA, et al. The longitudinal course of sleep timing and circadian preferences in adults with bipolar disorder. Bipolar Disord. 2015;17:392–402.
Steinan MK, Scott J, Lagerberg TV, Melle I, Andreassen OA, Vaaler AE, et al. Sleep problems in bipolar disorders: more than just insomnia. Acta Psychiatr Scand. 2016;133:368–77.
Gruber J, Harvey AG, Wang PW, Brooks JO III, Thase ME, Sachs GS, et al. Sleep functioning in relation to mood, function, and quality of life at entry to the systematic treatment enhancement program for bipolar disorder (STEP-BD). J Affect Disord. 2009;114:41–49.
Wirz-Justice A, Benedetti F. Perspectives in affective disorders: Clocks and sleep. Eur J Neurosci. 2019;51:346–65.
Gold AK, Kinrys G. Treating circadian rhythm disruption in bipolar disorder. Curr Psychiatry Rep. 2019;21:14.
Maruani J, Geoffroy PA. Bright light as a personalized precision treatment of mood disorders. Front Psychiatry. 2019;10:85.
Calabrese JR, Guelfi JD, Perdrizet-Chevallier C. Agomelatine bipolar study G. Agomelatine adjunctive therapy for acute bipolar depression: preliminary open data. Bipolar Disord. 2007;9:628–35.
Fornaro M, McCarthy MJ, De Berardis D, De Pasquale C, Tabaton M, Martino M, et al. Adjunctive agomelatine therapy in the treatment of acute bipolar II depression: a preliminary open label study. Neuropsychiatr Dis Treat. 2013;9:243–51.
Yatham LN, Vieta E, Goodwin GM, Bourin M, de Bodinat C, Laredo J, et al. Agomelatine or placebo as adjunctive therapy to a mood stabiliser in bipolar I depression: randomised double-blind placebo-controlled trial. Br J Psychiatry. 2016;208:78–86.
Mahableshwarkar AR, Calabrese JR, Macek TA, Budur K, Adefuye A, Dong X, et al. Efficacy and safety of sublingual ramelteon as an adjunctive therapy in the maintenance treatment of bipolar I disorder in adults: A phase 3, randomized controlled trial. J Affect Disord. 2017;221:275–82.
Zhou TH, Dang WM, Ma YT, Hu CQ, Wang N, Zhang GY, et al. Clinical efficacy, onset time and safety of bright light therapy in acute bipolar depression as an adjunctive therapy: a randomized controlled trial. J Affect Disord. 2018;227:90–96.
Sit DK, McGowan J, Wiltrout C, Diler RS, Dills JJ, Luther J, et al. Adjunctive bright light therapy for bipolar depression: a randomized double-blind placebo-controlled trial. Am J Psychiatry. 2018;175:131–9.
Phelps J. A powerful non-pharmacologic treatment for mania – virtually. Bipolar Disord. 2016;18:379–82.
Do MTH. Melanopsin and the intrinsically photosensitive retinal ganglion cells: biophysics to behavior. Neuron. 2019;104:205–26.
Kim KY, Rios LC, Le H, Perez AJ, Phan S, Bushong EA, et al. Synaptic specializations of melanopsin-retinal ganglion cells in multiple brain regions revealed by genetic label for light and electron microscopy. Cell Rep. 2019;29:628–44 e626.
Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–9.
Jones KA, Hatori M, Mure LS, Bramley JR, Artymyshyn R, Hong SP, et al. Small-molecule antagonists of melanopsin-mediated phototransduction. Nat Chem Biol. 2013;9:630–5.
Henriksen TE, Skrede S, Fasmer OB, Hamre B, Gronli J, Lund A. Blocking blue light during mania - markedly increased regularity of sleep and rapid improvement of symptoms: a case report. Bipolar Disord. 2014;16:894–8.
Henriksen TE, Skrede S, Fasmer OB, Schoeyen H, Leskauskaite I, Bjorke-Bertheussen J, et al. Blue-blocking glasses as additive treatment for mania: a randomized placebo-controlled trial. Bipolar Disord. 2016;18:221–32.
Acknowledgements
We would like to acknowledge helpful discussion and feedback from members of the Haggarty, Perlis, and Karmacharya laboratories, along with members of the Dauten Family Center for Bipolar Treatment Innovation at Massachusetts General Hospital. This work was supported through funding from the Stuart & Suzanne Steele MGH Research Scholars Program, NIH R01MH120227, NIH R01AT009144, NIH R01MH113858. This manuscript is dedicated to the compassionate staff of the Rockyview General Hospital and Foothills Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SJH was or is a member of the scientific advisory board of Rodin Therapeutics, Psy Therapeutics, Frequency Therapeutics, and Souvien Therapeutics, none of whom were involved in the present study. SJH has also received speaking or consulting fees from Amgen, AstraZeneca, Biogen, Merck, Regenacy Pharmaceuticals, Syros Pharmaceuticals, Juvenescence, as well as sponsored research or gift funding from AstraZeneca, JW Pharmaceuticals, and Vesigen unrelated to the content of this manuscript. RHP is a member of the scientific advisory board of Psy Therapeutics, Outermost Therapeutics, and Genomind, and reports consulting fees from Genomind, RID Ventures, Takeda, and Burrage Capital. He holds equity in Psy Therapeutics and Outermost Therapeutics. All of these are unrelated to the content of this manuscript. RK: None.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haggarty, S.J., Karmacharya, R. & Perlis, R.H. Advances toward precision medicine for bipolar disorder: mechanisms & molecules. Mol Psychiatry 26, 168–185 (2021). https://doi.org/10.1038/s41380-020-0831-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-0831-4
This article is cited by
-
Progress and Implications from Genetic Studies of Bipolar Disorder
Neuroscience Bulletin (2024)
-
A novel murine model of mania
Molecular Psychiatry (2023)
-
T cells: an emerging cast of roles in bipolar disorder
Translational Psychiatry (2023)
-
Genetic evidence for the “dopamine hypothesis of bipolar disorder”
Molecular Psychiatry (2023)
-
Impulsivity across severe mental disorders: a cross-sectional study of immune markers and psychopharmacotherapy
BMC Psychiatry (2023)