Abstract
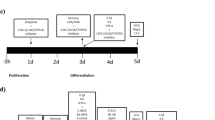
Evidence from epidemiological and laboratory studies, as well as randomized placebo-controlled trials, suggests supplementation with n-3 polyunsaturated fatty acids (PUFAs) may be efficacious for treatment of major depressive disorder (MDD). The mechanisms underlying n-3 PUFAs potential therapeutic properties remain unknown. There are suggestions in the literature that glial hypofunction is associated with depressive symptoms and that antidepressants may normalize glial function. In this study, induced pluripotent stem cells (iPSC)-derived neuronal stem cell lines were generated from individuals with MDD. Astrocytes differentiated from patient-derived neuronal stem cells (iNSCs) were verified by GFAP. Cells were treated with eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) or stearic acid (SA). During astrocyte differentiation, we found that n-3 PUFAs increased GFAP expression and GFAP positive cell formation. BDNF and GDNF production were increased in the astrocytes derived from patients subsequent to n-3 PUFA treatment. Stearic Acid (SA) treatment did not have this effect. CREB activity (phosphorylated CREB) was also increased by DHA and EPA but not by SA. Furthermore, when these astrocytes were treated with n-3 PUFAs, the cAMP antagonist, RP-cAMPs did not block n-3 PUFA CREB activation. However, the CREB specific inhibitor (666-15) diminished BDNF and GDNF production induced by n-3 PUFA, suggesting CREB dependence. Together, these results suggested that n-3 PUFAs facilitate astrocyte differentiation, and may mimic effects of some antidepressants by increasing production of neurotrophic factors. The CREB-dependence and cAMP independence of this process suggests a manner in which n-3 PUFA could augment antidepressant effects. These data also suggest a role for astrocytes in both MDD and antidepressant action.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60.
Kraus C, Kadriu B, Lanzenberger R, Zarate CA Jr., Kasper S. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry. 2019;9:127.
Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63.
Malhi GS, Das P, Mannie Z, Irwin L. Treatment-resistant depression: problematic illness or a problem in our approach? Br J Psychiatry. 2019;214:1–3.
Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–11.
Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, et al. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126:75–91.
Sanacora G, Banasr M. From pathophysiology to novel antidepressant drugs: glial Contributions to the pathology and treatment of mood disorders. Biol Psychiatry. 2013;73:172–9.
Wang Q, Jie W, Liu JH, Yang JM, Gao TM. An astroglial basis of major depressive disorder? An overview. Glia. 2017;65:1227–50.
Musazzi L, Rimland JM, Ieraci A, Racagni G, Domenici E, Popoli M. Pharmacological characterization of BDNF promoters I, II and IV reveals that serotonin and norepinephrine input is sufficient for transcription activation. Int J Neuropsychopharmacol. 2014;17:779–91.
Hisaoka-Nakashima K, Kajitani N, Kaneko M, Shigetou T, Kasai M, Matsumoto C, et al. Amitriptyline induces brain-derived neurotrophic factor (BDNF) mRNA expression through ERK-dependent modulation of multiple BDNF mRNA variants in primary cultured rat cortical astrocytes and microglia. Brain Res. 2016;1634:57–67.
Allaman I, Fiumelli H, Magistretti PJ, Martin JL. Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacol (Berl). 2011;216:75–84.
Quesseveur G, David DJ, Gaillard MC, Pla P, Wu MV, Nguyen HT, et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry. 2013;3:e253.
Burhani MD, Rasenick MM. Fish oil and depression: the skinny on fats. J Integr Neurosci. 2017;16(s1):S115–24.
Bai ZG, Bo A, Wu SJ, Gai QY, Chi I. Omega-3 polyunsaturated fatty acids and reduction of depressive symptoms in older adults: A systematic review and meta-analysis. J Affect Disord. 2018;241:241–8.
Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. omega-3 Fatty acids for major depressive disorder in adults: an abridged Cochrane review. BMJ Open. 2016;6:e010172.
Sublette ME, Galfalvy HC, Hibbeln JR, Keilp JG, Malone KM, Oquendo MA, et al. Polyunsaturated fatty acid associations with dopaminergic indices in major depressive disorder. Int J Neuropsychopharmacol. 2014;17:383–91.
Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21:71–9.
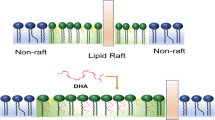
Czysz AH, Rasenick MM. G-protein signaling, lipid rafts and the possible sites of action for the antidepressant effects of n-3 polyunsaturated fatty acids. CNS Neurol Disord Drug Targets. 2013;12:466–73.
Soliman MA, Aboharb F, Zeltner N, Studer L. Pluripotent stem cells in neuropsychiatric disorders. Mol Psychiatry. 2017;22:1241–9.
Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22:374–85.
Vadodaria KC, Ji Y, Skime M, Paquola AC, Nelson T, Hall-Flavin D, et al. Altered serotonergic circuitry in SSRI-resistant major depressive disorder patient-derived neurons. Mol Psychiatry. 2019;24:808–18.
Zhao WN, Hylton NK, Wang J, Chindavong PS, Alural B, Kurtser I, et al. Activation of WNT and CREB signaling pathways in human neuronal cells in response to the Omega-3 fatty acid docosahexaenoic acid (DHA). Mol Cell Neurosci. 2019;99:103386.
Madison JM, Zhou F, Nigam A, Hussain A, Barker DD, Nehme R, et al. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol Psychiatry. 2015;20:703–17.
Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59:1144–50.
Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–9.
Desseilles M, Witte J, Chang TE, Iovieno N, Dording CM, Ashih H, et al. Assessing the adequacy of past antidepressant trials: a clinician's guide to the antidepressant treatment response questionnaire. J Clin Psychiatry. 2011;72:1152–4.
Brown HE, Freudenreich O, Fan X, Heard SO, Goff D, Petrides G, et al. Efficacy and tolerability of adjunctive intravenous sodium nitroprusside treatment for outpatients with schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2019;76:691–9.
Shaltouki A, Peng J, Liu Q, Rao MS, Zeng X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells. 2013;31:941–52.
Tian C, Liu Q, Ma K, Wang Y, Chen Q, Ambroz R, et al. Characterization of induced neural progenitors from skin fibroblasts by a novel combination of defined factors. Sci Rep. 2013;3:1345.
Cao W, Ma Z, Rasenick MM, Yeh S, Yu J. N-3 poly-unsaturated fatty acids shift estrogen signaling to inhibit human breast cancer cell growth. PLoS One. 2012;7:e52838.
Roy J, Lefkimmiatis K, Moyer MP, Curci S, Hofer AM. The {omega}-3 fatty acid eicosapentaenoic acid elicits cAMP generation in colonic epithelial cells via a ‘store-operated’ mechanism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G715–22.
Wray NH, Schappi JM, Singh H, Senese NB, Rasenick MM. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol Psychiatry. 2019;24:1833–43.
Sutkowski EM, Tang WJ, Broome CW, Robbins JD, Seamon KB. Regulation of forskolin interactions with type I, II, V, and VI adenylyl cyclases by Gs alpha. Biochemistry. 1994;33:12852–9.
Gittins RA, Harrison PJ. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J Affect Disord. 2011;133:328–32.
Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2015;20:320–8.
Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–12.
Kekesi KA, Juhasz G, Simor A, Gulyassy P, Szego EM, Hunyadi-Gulyas E, et al. Altered functional protein networks in the prefrontal cortex and amygdala of victims of suicide. PLoS One. 2012;7:e50532.
Kinoshita M, Hirayama Y, Fujishita K, Shibata K, Shinozaki Y, Shigetomi E, et al. Anti-depressant fluoxetine reveals its therapeutic effect via astrocytes. EBioMed. 2018;32:72–83.
Koyama Y, Egawa H, Osakada M, Baba A, Matsuda T. Increase by FK960, a novel cognitive enhancer, in glial cell line-derived neurotrophic factor production in cultured rat astrocytes. Biochem Pharm. 2004;68:275–82.
Sharma AN, da Costa e Silva BF, Soares JC, Carvalho AF, Quevedo J. Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: a comprehensive review of human studies. J Affect Disord. 2016;197:9–20.
Tsybko AS, Ilchibaeva TV, Popova NK. Role of glial cell line-derived neurotrophic factor in the pathogenesis and treatment of mood disorders. Rev Neurosci. 2017;28:219–33.
Matsuoka Y, Nishi D, Yonemoto N, Hamazaki K, Hamazaki T, Hashimoto K. Potential role of brain-derived neurotrophic factor in omega-3 Fatty Acid supplementation to prevent posttraumatic distress after accidental injury: an open-label pilot study. Psychother Psychosom. 2011;80:310–2.
Vines A, Delattre AM, Lima MM, Rodrigues LS, Suchecki D, Machado RB, et al. The role of 5-HT(1)A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: a possible antidepressant mechanism. Neuropharmacology. 2012;62:184–91.
Zemdegs J, Rainer Q, Grossmann CP, Rousseau-Ralliard D, Grynberg A, Ribeiro E, et al. Anxiolytic- and antidepressant-like effects of fish oil-enriched diet in brain-derived neurotrophic factor deficient mice. Front Neurosci. 2018;12:974.
Chen SJ, Kao CL, Chang YL, Yen CJ, Shui JW, Chien CS, et al. Antidepressant administration modulates neural stem cell survival and serotoninergic differentiation through bcl-2. Curr Neurovasc Res. 2007;4:19–29.
Hisaoka K, Maeda N, Tsuchioka M, Takebayashi M. Antidepressants induce acute CREB phosphorylation and CRE-mediated gene expression in glial cells: a possible contribution to GDNF production. Brain Res. 2008;1196:53–8.
Xue W, Wang W, Gong T, Zhang H, Tao W, Xue L, et al. PKA-CREB-BDNF signaling regulated long lasting antidepressant activities of Yueju but not ketamine. Sci Rep. 2016;6:26331.
Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–4.
Zhang L, Rasenick MM. Chronic treatment with escitalopram but not R-citalopram translocates Galpha(s) from lipid raft domains and potentiates adenylyl cyclase: a 5-hydroxytryptamine transporter-independent action of this antidepressant compound. J Pharm Exp Ther. 2010;332:977–84.
Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–8.
Yang C, Li T, Xue H, Wang L, Deng L, Xie Y, et al. Inhibition of necroptosis rescues SAH-induced synaptic impairments in hippocampus via CREB-BDNF pathway. Front Neurosci. 2018;12:990.
Chandley MJ, Szebeni K, Szebeni A, Crawford J, Stockmeier CA, Turecki G, et al. Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. J Psychiatry Neurosci. 2013;38:276–84.
Kang JX, Wan JB, He C. Concise review: regulation of stem cell proliferation and differentiation by essential fatty acids and their metabolites. Stem Cells. 2014;32:1092–8.
Das M, Das S. Docosahexaenoic acid (DHA) induced morphological differentiation of astrocytes is associated with transcriptional upregulation and endocytosis of beta2-AR. Mol Neurobiol. 2019;56:2685–702.
Popoli M. Agomelatine: innovative pharmacological approach in depression. CNS Drugs. 2009;23 (Suppl 2):27–34.
Castren E, Antila H. Neuronal plasticity and neurotrophic factors in drug responses. Mol Psychiatry. 2017;22:1085–95.
Park YM, Lee BH. Alterations in serum BDNF and GDNF levels after 12 weeks of antidepressant treatment in female outpatients with major depressive disorder. Psychiatry Investig. 2018;15:818–23.
Bazinet RP, Metherel AH, Chen CT, Shaikh SR, Nadjar A, Joffre C, et al. Brain eicosapentaenoic acid metabolism as a lead for novel therapeutics in major depression. Brain Behav Immun. 2020;85:21–8.
Hallahan B, Ryan T, Hibbeln JR, Murray IT, Glynn S, Ramsden CE, et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016;209:192–201.
Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9:190.
Jin Y, Park Y. N-3 polyunsaturated fatty acids and 17beta-estradiol injection induce antidepressant-like effects through regulation of serotonergic neurotransmission in ovariectomized rats. J Nutr Biochem. 2015;26:970–7.
Liu JH, Wu ZF, Sun J, Jiang L, Jiang S, Fu WB. Role of AC-cAMP-PKA cascade in antidepressant action of electroacupuncture treatment in rats. Evid Based Complement Altern Med. 2012;2012:932414.
Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, et al. A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology. 1992;26:59–64.
Fujimaki K, Morinobu S, Duman RS. Administration of a cAMP phosphodiesterase 4 inhibitor enhances antidepressant-induction of BDNF mRNA in rat hippocampus. Neuropsychopharmacology. 2000;22:42–51.
Itoh T, Tokumura M, Abe K. Effects of rolipram, a phosphodiesterase 4 inhibitor, in combination with imipramine on depressive behavior, CRE-binding activity and BDNF level in learned helplessness rats. Eur J Pharm. 2004;498:135–42.
Gurney ME, Nugent RA, Mo X, Sindac JA, Hagen TJ, Fox D 3rd, et al. Design and synthesis of selective phosphodiesterase 4D (PDE4D) allosteric inhibitors for the treatment of fragile X syndrome and other brain disorders. J Med Chem. 2019;62:4884–901.
Baecker PA, Lee WH, Verity AN, Eglen RM, Johnson RM. Characterization of a promoter for the human glial cell line-derived neurotrophic factor gene. Brain Res Mol Brain Res. 1999;69:209–22.
Acknowledgements
This research is supported by NIH R01AT009169 (MMR, JZY) and VA Merit Award BX00149 (MMR) and NIH R41MH113398 (MMR). Patient cell line collection and derivation were supported by NIH P50MH106933 and NIH R01AT009144 (RHP, SDS, JW). MMR is a VA Research Career Scientist BX004475.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
MMR has received research support from Lundbeck SA and consulting fee from Otsuka, INC. He also has ownership interest in PAX Neuroscience.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, JZ., Wang, J., Sheridan, S.D. et al. N-3 polyunsaturated fatty acids promote astrocyte differentiation and neurotrophin production independent of cAMP in patient-derived neural stem cells. Mol Psychiatry 26, 4605–4615 (2021). https://doi.org/10.1038/s41380-020-0786-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-0786-5
This article is cited by
-
Protective effects of docosahexaenoic acid supplementation on cognitive dysfunction and hippocampal synaptic plasticity impairment induced by early postnatal PM2.5 exposure in young rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Impact of membrane lipid polyunsaturation on dopamine D2 receptor ligand binding and signaling
Molecular Psychiatry (2023)
-
The stress–Wnt-signaling axis: a hypothesis for attention-deficit hyperactivity disorder and therapy approaches
Translational Psychiatry (2020)