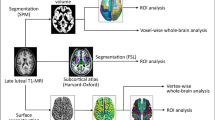
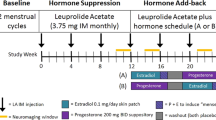
Abstract
Gonadal hormones influence neuronal organization and plasticity. Yet the consequences of altering their concentrations by administering contraceptive agents, which are used by most reproductive-age women in the United States, are unclear. Cross-sectional studies have found both larger and smaller cortical regions alongside a variety of mood alterations in women who use oral contraceptive pills (OCPs) compared to naturally-cycling women. The goal of this study, therefore, was to determine whether there is an effect of OCPs on MRI measures of prefrontal cortical brain structure that may influence regulation of mood. We performed a double-blind, placebo-controlled, randomized crossover study comparing effects of OCPs (0.15 mg levonorgestrel + 0.30 μg ethinyl estradiol) vs placebo (N = 26) on MRI measures of prefrontal cortical thickness and on mood, as indicated by self-report on the Daily Record of Severity of Problems, which also includes one item related to somatic symptoms. MRI measures that reflect cortical thickness were smaller bilaterally in the pars triangularis and in the pars opercularis and frontal pole of the right hemisphere during the OCP arm vs. placebo. Only the effect in the right pars triangularis survived multiple comparisons correction. Right pars triangularis MRI measures of cortical thickness were not related to mood symptoms, but negatively correlated across conditions with severity of somatic symptoms on the DSRP. The somatic symptoms and MRI measures may be independently related to the actions of steroid hormones in OCPs, with OCPs simultaneously inducing both more effects on MRI measures of cortical thickness and somatic symptoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Taggart TC, Eaton NR, Keyes KM, Hammett JF, Ulloa EC. Oral contraceptive use is associated with greater mood stability and higher relationship satisfaction. Neurol Psychiatry Brain Res. 2018;30:154–62.
Hamstra DA, de Kloet ER, de Rover M, Van der Does W. Oral contraceptives positively affect mood in healthy PMS-free women: a longitudinal study. J Psychosom Res. 2017;103:119–26.
Cheslack-Postava K, Keyes KM, Lowe SR, Koenen KC. Oral contraceptive use and psychiatric disorders in a nationally representative sample of women. Arch Women’s Ment Health. 2015;18:103–11.
Keyes KM, Cheslack-Postava K, Westhoff C, Heim CM, Haloossim M, Walsh K, et al. Association of hormonal contraceptive use with reduced levels of depressive symptoms: a national study of sexually active women in the United States. Am J Epidemiol. 2013;178:1378–88.
Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73:1154–62.
Skovlund CW, Mørch LS, Kessing LV, Lange T, Lidegaard Ø. Association of hormonal contraception with suicide attempts and suicides. Am J psychiatry. 2018;175:336–42.
de Wit AE, Booij SH, Giltay EJ, Joffe H, Schoevers RA, Oldehinkel AJ. Association of use of oral contraceptives with depressive symptoms among adolescents and young women. JAMA Psychiatry. 2020;77:52–9.
Shakerinejad G, Hidarnia A, Motlagh ME, Karami K, Niknami S, Montazeri A. Factors predicting mood changes in oral contraceptive pill users. Reprod Health. 2013;10:45.
Westhoff CL, Heartwell S, Edwards S, Zieman M, Stuart G, Cwiak C, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412. e411-412. e417
Wiebe ER, Brotto LA, MacKay J. Characteristics of women who experience mood and sexual side effects with use of hormonal contraception. J Obstet Gynaecol Can. 2011;33:1234–40.
Lobo RA, Stanczyk FZ. New knowledge in the physiology of hormonal contraceptives. Am J Obstet Gynecol. 1994;170:1499–507.
Collins DC. Sex hormone receptor binding, progestin selectivity, and the new oral contraceptives. Am J Obstet Gynecol. 1994;170:1508–13.
Fleischman DS, Navarrete CD, Fessler DMT. Oral contraceptives suppress ovarian hormone production. Psychol Sci. 2010;21:750–2.
Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 2010;1348:55–62.
Sharma R, Smith SA, Boukina N, Dordari A, Mistry A, Taylor BC, et al. Use of the birth control pill affects stress reactivity and brain structure and function. Hormones Behav. 2020;124:104783.
Petersen N, Touroutoglou A, Andreano JM, Cahill L. Oral contraceptive pill use is associated with localized decreases in cortical thickness. Hum brain Mapp. 2015;36:2644–54.
Pletzer B, Kronbichler M, Kerschbaum H. Differential effects of androgenic and anti-androgenic progestins on fusiform and frontal gray matter volume and face recognition performance. Brain Res. 2015;1596:108–15.
Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA. 2001;98:13391–5.
Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, et al. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–43.
Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Hormones Behav. 1998;34:140–8.
Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–22.
Haraguchi S, Sasahara K, Shikimi H, Honda S-i, Harada N, Tsutsui K. Estradiol promotes purkinje dendritic growth, spinogenesis, and synaptogenesis during neonatal life by inducing the expression of BDNF. Cerebellum. 2012;11:416–7.
Fester L, Rune GM. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Res. 2015;1621:162–9.
Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. β-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–20.
Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306.
Naftolin F, Mor G, Horvath TL, Luquin S, Fajer AB, Kohen F, et al. Synaptic remodeling in the arcuate nucleus during the estrous cycle is induced by estrogen and precedes the preovulatory gonadotropin surge. Endocrinology. 1996;137:5576–80.
Parducz A, Perez J, Garcia-Segura L. Estradiol induces plasticity of GABAergic synapses in the hypothalamus. Neuroscience. 1993;53:395–401.
Inghilleri M, Conte A, Curra A, Frasca V, Lorenzano C, Berardelli A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin Neurophysiol. 2004;115:1063–8.
Lee S, Chung SW, Rogasch NC, Thomson CJ, Worsley RN, Kulkarni J, et al. The influence of endogenous estrogen on transcranial direct current stimulation: a preliminary study. Eur J Neurosci. 2018;48:2001–12.
Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Effects of ovarian hormones on human cortical excitability. Ann Neurol. 2002;51:599–603.
Bixo M, Andersson A, Winblad B, Purdy RH, Bäckström T. Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–8.
Bixo M, Bäckström T, Winblad B, Andersson A. Estradiol and testosterone in specific regions of the human female brain in different endocrine states. J Steroid Biochem Mol Biol. 1995;55:297–303.
Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab. 2005;90:2851–4.
Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–58.
Videbech P, Yttri JE. The effect of antidepressants on brain volume. Ugeskr Laege. 2019;181:38.
Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1.
Schwartz JL, Creinin MD, Pymar HC, Reid L. Predicting risk of ovulation in new start oral contraceptive users. Obstet Gynecol. 2002;99:177–82.
Danforth DR, Hodgen GD. “Sunday start” multiphasic oral contraception: ovulation prevention and delayed follicular atresia in primates. Contraception. 1989;39:321–30.
Killick S, Eyong E, Elstein M.Ovarian follicular development in oral contraceptive cycles** Supported by Wyeth International, Philadelphia, Pennsylvania.Fertil Steril. 1987;48:409–13.
Hoogland HJ, Skouby SO. Ultrasound evaluation of ovarian activity under oral contraceptives. Contraception. 1993;47:583–90.
Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Women’s Ment health. 2006;9:41–9.
Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22.
Klapwijk ET, Van De Kamp F, Van Der Meulen M, Peters S, Wierenga LM. Qoala-T: a supervised-learning tool for quality control of FreeSurfer segmented MRI data. Neuroimage. 2019;189:116–29.
Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2009;20:534–48.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol). 1995;57:289–300.
Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–502.
Pletzer B. Sex hormones and gender role relate to grey matter volumes in sexually dimorphic brain areas. Front Neurosci. 2019;13:592.
Hantsoo L, Epperson CN. Allopregnanolone in premenstrual dysphoric disorder (PMDD): evidence for dysregulated sensitivity to GABA-A receptor modulating neuroactive steroids across the menstrual cycle. Neurobiol Stress. 2020;12:100213.
Walton N, Maguire J. Allopregnanolone-based treatments for postpartum depression: Why/how do they work? Neurobiol Stress. 2019;11:100198.
Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S-i, Harada N, et al. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci. 2007;27:7408–17.
Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Böhm J, Jarry H, et al. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J steroid Biochem Mol Biol. 2012;131:24–29.
Pletzer B, Harris T, Hidalgo-Lopez E. Previous contraceptive treatment relates to grey matter volumes in the hippocampus and basal ganglia. Sci Rep. 2019;9:11003.
Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-11. N Engl J Med. 2016;374:843–52.
Nations U. Contraceptive use by method 2019: data booklet (ST/ESA/SER.A/435). Department of Economic and Social Affairs, Population Division 2019.
Acknowledgements
This work was supported by NIDA (R21DA040168 to EDL), the Marjorie Greene Family Trust, and the Thomas P and Katherine K Pike Chair in Addiction Studies (EDL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Petersen, N., Kearley, N.W., Ghahremani, D.G. et al. Effects of oral contraceptive pills on mood and magnetic resonance imaging measures of prefrontal cortical thickness. Mol Psychiatry 26, 917–926 (2021). https://doi.org/10.1038/s41380-020-00990-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00990-2
This article is cited by
-
Towards a more comprehensive neuroscience of hormonal contraceptives
Nature Neuroscience (2023)