Abstract
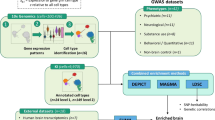
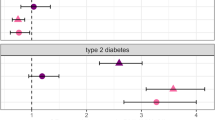
Major mental illnesses such as schizophrenia (SZ) and bipolar disorder (BP) frequently accompany metabolic conditions, but their relationship is still unclear, in particular at the mechanistic level. We implemented an approach of “from population to neuron”, combining population-based epidemiological analysis with neurobiological experiments using cell and animal models based on a hypothesis built from the epidemiological study. We characterized high-quality population data, olfactory neuronal cells biopsied from patients with SZ or BP, and healthy subjects, as well as mice genetically modified for insulin signaling. We accessed the Danish Registry and observed (1) a higher incidence of diabetes in people with SZ or BP and (2) higher incidence of major mental illnesses in people with diabetes in the same large cohort. These epidemiological data suggest the existence of common pathophysiological mediators in both diabetes and major mental illnesses. We hypothesized that molecules associated with insulin resistance might be such common mediators, and then validated the hypothesis by using two independent sets of olfactory neuronal cells biopsied from patients and healthy controls. In the first set, we confirmed an enrichment of insulin signaling-associated molecules among the genes that were significantly different between SZ patients and controls in unbiased expression profiling data. In the second set, olfactory neuronal cells from SZ and BP patients who were not pre-diabetic or diabetic showed reduced IRS2 tyrosine phosphorylation upon insulin stimulation, indicative of insulin resistance. These cells also displayed an upregulation of IRS1 protein phosphorylation at serine-312 at baseline (without insulin stimulation), further supporting the concept of insulin resistance in olfactory neuronal cells from SZ patients. Finally, Irs2 knockout mice showed an aberrant response to amphetamine, which is also observed in some patients with major mental illnesses. The bi-directional relationships between major mental illnesses and diabetes suggest that there may be common pathophysiological mediators associated with insulin resistance underlying these mental and physical conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Weissman MM, Brown AS, Talati A. Translational epidemiology in psychiatry: linking population to clinical and basic sciences. Arch Gen Psychiatry. 2011;68:600–8.
Morris JN. Uses of epidemiology. 3rd ed. Edinburgh: Churchill Livingstone; 1975.
Torrey EF, Torrey BB, Peterson MR. Seasonality of schizophrenic births in the United States. Arch Gen Psychiatry. 1977;34:1065–70.
Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–80.
Curtis VA, van Os J, Murray RM. The Kraepelinian dichotomy: evidence from developmental and neuroimaging studies. J Neuropsychiatry Clin Neurosci. 2000;12:398–405.
Neale BM, Sklar P. Genetic analysis of schizophrenia and bipolar disorder reveals polygenicity but also suggests new directions for molecular interrogation. Curr Opin Neurobiol. 2015;30:131–8.
Vancampfort D, Correll CU, Galling B, Probst M, De Hert M, Ward PB, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15:166–74.
Liao CH, Chang CS, Wei WC, Chang SN, Liao CC, Lane HY, et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia: a population-based study. Schizophr Res. 2011;126:110–6.
Bai YM, Su TP, Chen MH, Chen TJ, Chang WH. Risk of developing diabetes mellitus and hyperlipidemia among patients with bipolar disorder, major depressive disorder, and schizophrenia: a 10-year nationwide population-based prospective cohort study. J Affect Disord. 2013;150:57–62.
Enger C, Jones ME, Kryzhanovskaya L, Doherty M, McAfee AT. Risk of developing diabetes and dyslipidemia among adolescents with bipolar disorder or schizophrenia. Int J Adolesc Med Health. 2013;25:3–11.
Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune diseases and psychosis. Biol Psychiatry. 2014;75:300–6.
Schwartz TL, Nihalani N, Jindal S, Virk S, Jones N. Psychiatric medication-induced obesity: a review. Obes Rev. 2004;5:115–21.
Fernandez-Egea E, Bernardo M, Donner T, Conget I, Parellada E, Justicia A, et al. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br J Psychiatry. 2009;194:434–8.
Garcia-Rizo C, Kirkpatrick B, Fernandez-Egea E, Oliveira C, Bernardo M. Abnormal glycemic homeostasis at the onset of serious mental illnesses: a common pathway. Psychoneuroendocrinology. 2016;67:70–5.
Greenhalgh AM, Gonzalez-Blanco L, Garcia-Rizo C, Fernandez-Egea E, Miller B, Arroyo MB, et al. Meta-analysis of glucose tolerance, insulin, and insulin resistance in antipsychotic-naive patients with nonaffective psychosis. Schizophr Res. 2017;179:57–63.
Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53:441–9.
Munk-Jorgensen P, Mortensen PB. The Danish psychiatric central register. Dan Med Bull. 1997;44:82–4.
Carstensen B, Kristensen JK, Marcussen MM, Borch-Johnsen K. The national diabetes register. Scand J Public Health. 2011;39:58–61.
Evgrafov OV, Armoskus C, Wrobel BB, Spitsyna VN, Souaiaia T, Herstein JS, et al. Gene expression in patient-derived neural progenitors implicates WNT5A signaling in the etiology of schizophrenia. Biol Psychiatry. 2020;88:236–47.
Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A, et al. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry. 2013;18:740–2.
Sumitomo A, Yukitake H, Hirai K, Horike K, Ueta K, Chung Y, et al. Ulk2 controls cortical excitatory-inhibitory balance via autophagic regulation of p62 and GABAA receptor trafficking in pyramidal neurons. Hum Mol Genet. 2018;27:3165–76.
Wu J, Tseng YD, Xu CF, Neubert TA, White MF, Hubbard SR. Structural and biochemical characterization of the KRLB region in insulin receptor substrate-2. Nat Struct Mol Biol. 2008;15:251–8.
Emdal KB, Pedersen AK, Bekker-Jensen DB, Lundby A, Claeys S, De Preter K, et al. Integrated proximal proteomics reveals IRS2 as a determinant of cell survival in ALK-driven neuroblastoma. Sci Signal. 2018;11.
Valverde AM, Burks DJ, Fabregat I, Fisher TL, Carretero J, White MF, et al. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes. 2003;52:2239–48.
Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–72.
Kozuka T, Omori Y, Watanabe S, Tarusawa E, Yamamoto H, Chaya T, et al. miR-124 dosage regulates prefrontal cortex function by dopaminergic modulation. Sci Rep. 2019;9:3445.
Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA. 2007;104:14501–6.
Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–9.
Sumitomo A, Horike K, Hirai K, Butcher N, Boot E, Sakurai T, et al. A mouse model of 22q11.2 deletions: molecular and behavioral signatures of Parkinson’s disease and schizophrenia. Sci Adv. 2018;4:eaar6637.
Hall DA, Stanis JJ, Marquez, Avila H, Gulley JM. A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: evidence for qualitative differences in behavior. Psychopharmacology. 2008;195:469–78.
Lavoie J, Sawa A, Ishizuka K. Application of olfactory tissue and its neural progenitors to schizophrenia and psychiatric research. Curr Opin Psychiatry. 2017;30:176–83.
Rhie SK, Schreiner S, Witt H, Armoskus C, Lay FD, Camarena A, et al. Using 3D epigenomic maps of primary olfactory neuronal cells from living individuals to understand gene regulation. Sci Adv. 2018;4:eaav8550.
Namkung H, Lee BJ, Sawa A. Causal inference on pathophysiological mediators in psychiatry. Cold Spring Harb Symp Quant Biol. 2018;83:17–23.
White MF. IRS2 integrates insulin/IGF1 signalling with metabolism, neurodegeneration and longevity. Diabetes Obes Metab. 2014;16 Suppl 1:4–15.
Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–91.
White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1.
Costello DA, Claret M, Al-Qassab H, Plattner F, Irvine EE, Choudhury AI, et al. Brain deletion of insulin receptor substrate 2 disrupts hippocampal synaptic plasticity and metaplasticity. PLoS ONE. 2012;7:e31124.
Howell KR, Floyd K, Law AJ. PKBgamma/AKT3 loss-of-function causes learning and memory deficits and deregulation of AKT/mTORC2 signaling: Relevance for schizophrenia. PLoS ONE. 2017;12:e0175993.
Wang W, Tanokashira D, Fukui Y, Maruyama M, Kuroiwa C, Saito T, et al. Serine phosphorylation of IRS1 correlates with Abeta-unrelated memory deficits and elevation in Abeta level prior to the onset of memory decline in AD. Nutrients. 2019;11.
Martin ED, Sanchez-Perez A, Trejo JL, Martin-Aldana JA, Cano Jaimez M, Pons S. et al. IRS-2 deficiency impairs NMDA receptor-dependent long-term potentiation. Cereb Cortex. 2012;22:1717–27.
Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S. et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–4.
Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 2015;3:461–71.
Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology. 1987;91:415–33.
Crawley JN. What’s wrong with my mouse?: Behavioral phenotyping of transgenic and knockout mice. 2nd ed: Hoboken, New Jersey: Wiley-Liss; 2007. p. 544.
Zhu J, Zhang S, Cai H, Wang C, Yu Y. Common and distinct functional stability abnormalities across three major psychiatric disorders. Neuroimage Clin. 2020;27:102352.
Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–84.
Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med. 2017;9.
Sedlak TW, Nucifora LG, Koga M, Shaffer LS, Higgs C, Tanaka T, et al. Sulforaphane augments glutathione and influences brain metabolites in human subjects: a clinical pilot study. Mol Neuropsychiatry. 2018;3:214–22.
Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, Talalay P, et al. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci USA. 2014;111:15550–5.
Shiina A, Kanahara N, Sasaki T, Oda Y, Hashimoto T, Hasegawa T, et al. An open study of sulforaphane-rich broccoli sprout extract in patients with schizophrenia. Clin Psychopharmacol Neurosci. 2015;13:62–7.
Perkins DO, Jeffries CD, Do KQ. Potential roles of redox dysregulation in the development of schizophrenia. Biol Psychiatry. 2020;88:326–36.
Houghton CA. Sulforaphane: Its “Coming of Age” as a clinically relevant nutraceutical in the prevention and treatment of chronic disease. Oxid Med Cell Longev. 2019;2019:2716870.
Alvarez-Arellano L, Gonzalez-Garcia N, Salazar-Garcia M, Corona JC. Antioxidants as a potential target against inflammation and oxidative stress in attention-deficit/hyperactivity disorder. Antioxidants. 2020;9.
Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann NY Acad Sci. 2017;1391:20–34.
Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93.
Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–83.
Lee SH, Zabolotny JM, Huang H, Lee H, Kim YB. Insulin in the nervous system and the mind: functions in metabolism, memory, and mood. Mol Metab. 2016;5:589–601.
Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16:59–65.
Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatry. 2016;21:10–28.
Badiola N, Suarez-Calvet M, Lleo A. Tau phosphorylation and aggregation as a therapeutic target in tauopathies. CNS Neurol Disord Drug Targets. 2010;9:727–40.
Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118:53–69.
Rajkumar AP, Horsdal HT, Wimberley T, Cohen D, Mors O, Borglum AD, et al. Endogenous and antipsychotic-related risks for diabetes mellitus in young people with schizophrenia: A Danish Population-Based Cohort Study. Am J Psychiatry. 2017;174:686–94.
Acknowledgements
We thank Yukiko Y. Lema for organizing the manuscript and figures, Drs. Melissa A. Landek-Salgado and Nao J. Gamo for critical reading of the manuscript. AS and KI are supported by the National Institute of Mental Health MH-105660 and Maryland Stem Cell Research Fund. AS is also supported by MH-094268 Silvio O. Conte center, MH-092443, and MH-107730, as well as foundation grants from Stanley, S-R/RUSK, BBRF. TS is supported by Kakenhi from the Ministry of Education, Culture, Sports, Science and Technology in Japan, and was funded in part by the Takeda and Kyoto University Basic and Clinical Research Project for CNS Drugs (supported in part by Takeda Pharmaceutical Co. Ltd). TML was supported by an unrestricted grant from the Stanley Medical Research Institute. PBM was supported by grants from the Danish Strategic Research Council, the Faculty of Social Sciences at Aarhus University, the Lundbeck Foundation, the Stanley Medical Research Institute, and a European Research Council advanced grant (GA 2948338). WWE was supported by NIMH grant 1R34MH007760-01.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Takayanagi, Y., Ishizuka, K., Laursen, T.M. et al. From population to neuron: exploring common mediators for metabolic problems and mental illnesses. Mol Psychiatry 26, 3931–3942 (2021). https://doi.org/10.1038/s41380-020-00939-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00939-5
This article is cited by
-
Inflammation-related pathology in the olfactory epithelium: its impact on the olfactory system in psychotic disorders
Molecular Psychiatry (2024)
-
Sex-specific involvement of the Notch–JAG pathway in social recognition
Translational Psychiatry (2022)
-
Temporally ordered associations between type 2 diabetes and brain disorders – a Danish register-based cohort study
BMC Psychiatry (2022)