Abstract
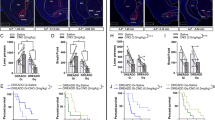
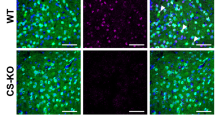
Anorexia nervosa (AN) is an eating disorder observed predominantly in women and girls that is characterized by a low body-mass index, hypophagia, and hyperactivity. Activity-based anorexia (ABA), which refers to the weight loss, hypophagia, and hyperactivity exhibited by rodents exposed to both running wheels and scheduled fasting, provides a model for aspects of AN. Increased dopamine D2/D3 receptor binding in the anteroventral striatum has been reported in AN patients. We virally overexpressed D2Rs on nucleus accumbens core (D2R-OENAc) neurons that endogenously express D2Rs, and tested mice of both sexes in the open field test, ABA paradigm, and intraperitoneal glucose tolerance test (IGTT). D2R-OENAc did not alter baseline body weight, but increased locomotor activity in the open field across both sexes. During constant access to food and running wheels, D2R-OENAc mice of both sexes increased food intake and ran more than controls. However, when food was available only 7 h a day, only female D2R-OENAc mice rapidly lost 25% of their initial body weight, reduced food intake, and substantially increased wheel running. Surprisingly, female D2R-OENAc mice also rapidly lost 25% of their initial body weight during scheduled fasting without wheel access and showed no changes in food intake. In contrast, male D2R-OENAc mice maintained body weight during scheduled fasting. D2R-OENAc mice of both sexes also showed glucose intolerance in the IGTT. In conclusion, D2R-OENAc alters glucose metabolism in both sexes but drives robust weight loss only in females during scheduled fasting, implicating metabolic mechanisms in this sexually dimorphic effect.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Attia E. Anorexia nervosa: current status and future directions. Annu Rev Med. 2010;61:425–35.
Schaumberg K, Welch E, Breithaupt L, Hubel C, Baker JH, Munn-Chernoff MA, et al. The science behind the Academy for Eating Disorders' nine truths about eating disorders. Eur Eat Disord Rev. 2017;25:432–50.
Watson HJ, Yilmaz Z, Thornton LM, Hubel C, Coleman JRI, Gaspar HA, et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. 2019;51:1207–14.
Duncan L, Yilmaz Z, Gaspar H, Walters R, Goldstein J, Anttila V, et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am J Psychiatry. 2017;174:850–8.
Hall JF, Hanford PV. Activity as a function of a restricted feeding schedule. J Comp Physiol Psychol. 1954;47:362–3.
Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol. 1967;64:414–21.
Burden VR, White BD, Dean RG, Martin RJ. Activity of the hypothalamic-pituitary-adrenal axis is elevated in rats with activity-based anorexia. J Nutr. 1993;123:1217–25.
Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav Neurosci. 1999;113:1080–9.
Klenotich SJ, Dulawa SC. The activity-based anorexia mouse model. Methods Mol Biol. 2012;829:377–93.
Pare WP, Vincent GP, Isom KE, Reeves JM. Sex differences and incidence of activity-stress ulcers in the rat. Psychol Rep. 1978;43:591–4.
Kaye WH, Frank GK, McConaha C. Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology. 1999;21:503–6.
Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58:908–12.
Broft A, Slifstein M, Osborne J, Kothari P, Morim S, Shingleton R, et al. Striatal dopamine type 2 receptor availability in anorexia nervosa. Psychiatry Res. 2015;233:380–7.
Frank GK, Shott ME, Hagman JO, Schiel MA, DeGuzman MC, Rossi B. The partial dopamine D2 receptor agonist aripiprazole is associated with weight gain in adolescent anorexia nervosa. Int J Eat Disord. 2017;50:447–50.
Klenotich SJ, Ho EV, McMurray MS, Server CH, Dulawa SC. Dopamine D2/3 receptor antagonism reduces activity-based anorexia. Transl Psychiatry. 2015;5:e613.
Foldi CJ, Milton LK, Oldfield BJ. The role of mesolimbic reward neurocircuitry in prevention and rescue of the activity-based anorexia (ABA) phenotype in rats. Neuropsychopharmacology. 2017;42:2292–300.
Gallo EF, Meszaros J, Sherman JD, Chohan MO, Teboul E, Choi CS, et al. Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat Commun. 2018;9:1086.
Gallo EF, Salling MC, Feng B, Moron JA, Harrison NL, Javitch JA, et al. Upregulation of dopamine D2 receptors in the nucleus accumbens indirect pathway increases locomotion but does not reduce alcohol consumption. Neuropsychopharmacology. 2015;40:1609–18.
Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature. 2016;531:642–6.
Macpherson T, Morita M, Wang Y, Sasaoka T, Sawa A, Hikida T. Nucleus accumbens dopamine D2-receptor expressing neurons control behavioral flexibility in a place discrimination task in the IntelliCage. Learn Mem. 2016;23:359–64.
Tchanturia K, Liao PC, Uher R, Lawrence N, Treasure J, Campbell IC. An investigation of decision making in anorexia nervosa using the Iowa gambling task and skin conductance measurements. J Int Neuropsychol Soc. 2007;13:635–41.
Giannunzio V, Degortes D, Tenconi E, Collantoni E, Solmi M, Santonastaso P, et al. Decision-making impairment in anorexia nervosa: New insights into the role of age and decision-making style. Eur Eat Disord Rev. 2018;26:302–14.
Tenconi E, Degortes D, Clementi M, Collantoni E, Pinato C, Forzan M, et al. Clinical and genetic correlates of decision making in anorexia nervosa. J Clin Exp Neuropsychol. 2016;38:327–37.
Friederich HC, Herzog W. Cognitive-behavioral flexibility in anorexia nervosa. Curr Top Behav Neurosci. 2011;6:111–23.
Zastrow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry. 2009;166:608–16.
Jiao J, Opal MD, Dulawa SC. Gestational environment programs adult depression-like behavior through methylation of the calcitonin gene-related peptide gene. Mol Psychiatry. 2012;18:1273–80.
Klenotich SJ, Seiglie MP, McMurray MS, Roitman JD, Le Grange D, Dugad P, et al. Olanzapine, but not fluoxetine, treatment increases survival in activity-based anorexia in mice. Neuropsychopharmacology. 2012;37:1620–31.
Michaelides M, Miller ML, DiNieri JA, Gomez JL, Schwartz E, Egervari G, et al. Dopamine D2 receptor signaling in the nucleus accumbens comprises a metabolic-cognitive brain interface regulating metabolic components of glucose reinforcement. Neuropsychopharmacology. 2017;42:2365–76.
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–85.
Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Meszaros J, et al. Evidence against dopamine D1/D2 receptor heteromers. Mol Psychiatry. 2015;20:1373–85.
Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–6.
Collins AL, Aitken TJ, Huang IW, Shieh C, Greenfield VY, Monbouquette HG et al. Nucleus accumbens cholinergic interneurons oppose cue-motivated behavior. Biol Psychiatry. 2019;86:388–96.
Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90.
Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–4.
Tecuapetla F, Jin X, Lima SQ, Costa RM. Complementary contributions of striatal projection pathways to action initiation and execution. Cell. 2016;166:703–15.
Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S, et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81:153–64.
Durieux PF, Schiffmann SN, de Kerchove, d'Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J. 2012;31:640–53.
Carvalho Poyraz F, Holzner E, Bailey MR, Meszaros J, Kenney L, Kheirbek MA, et al. Decreasing striatopallidal pathway function enhances motivation by energizing the initiation of goal-directed action. J Neurosci. 2016;36:5988–6001.
Dobbs LK, Kaplan AR, Lemos JC, Matsui A, Rubinstein M, Alvarez VA. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron. 2016;90:1100–13.
Dobbs LK, Kaplan AR, Bock R, Phamluong K, Shin JH, Bocarsly ME, et al. D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors. Neuropsychopharmacology. 2019;44:805–16.
Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–42.
Yttri EA, Dudman JT. Opponent and bidirectional control of movement velocity in the basal ganglia. Nature. 2016;533:402–6.
Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41.
Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–2.
Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8.
Pak K, Shin HK, Kim EJ, Lee JH, Lyoo CH, Son J, et al. Weight loss is associated with rapid striatal dopaminergic degeneration in Parkinson's disease. Parkinsonism Relat Disord. 2018;51:67–72.
Ferrario CR, Labouebe G, Liu S, Nieh EH, Routh VH, Xu S, et al. Homeostasis meets motivation in the battle to control food intake. J Neurosci. 2016;36:11469–81.
Morley LA, Gomez TH, Goldman JL, Flores R, Robinson MA. Accuracy of 5 point-of-care glucometers in C57BL/6J mice. J Am Assoc Lab Anim Sci. 2018;57:44–50.
Yasuhara D, Naruo T, Nagai N, Muranaga T, Nakahara T, Tanaka M, et al. Glucose tolerance predicts short-term refeeding outcome in females with anorexia nervosa. Psychosom Med. 2005;67:669–76.
Warren MP, Vande Wiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol. 1973;117:435–49.
Drossman DA, Ontjes DA, Heizer WD. Anorexia-Nervosa. Gastroenterology. 1979;77:1115–31.
Scheen AJ, Castillo M, Lefebvre PJ. Insulin sensitivity in anorexia nervosa: a mirror image of obesity? Diabetes Metab Rev. 1988;4:681–90.
Kumai M, Tamai H, Fujii S, Nakagawa T, Aoki TT. Glucagon secretion in anorexia nervosa. Am J Clin Nutr. 1988;47:239–42.
Nozaki T, Tamai H, Matsubayashi S, Komaki G, Kobayashi N, Nakagawa T. Insulin response to intravenous glucose in patients with anorexia nervosa showing low insulin response to oral glucose. J Clin Endocrinol Metab. 1994;79:217–22.
Lindfors C, Katz A, Selander L, Johansen JE, Marconi G, Schalling M, et al. Glucose intolerance and pancreatic beta-cell dysfunction in the anorectic anx/anx mouse. Am J Physiol Endocrinol Metab. 2015;309:E418–27.
Tahara A, Matsuyama-Yokono A, Nakano R, Someya Y, Shibasaki M. Effects of antidiabetic drugs on glucose tolerance in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic mice. Horm Metab Res. 2008;40:880–6.
Lee TJ, Kinzig KP. Repeated adolescent activity-based anorexia influences central estrogen signaling and adulthood anxiety-like behaviors in rats. Physiol Behav. 2017;171:199–206.
Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res Mol Brain Res. 2003;118:82–90.
Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93.
Zhang JP, Xu Q, Yuan XS, Cherasse Y, Schiffmann SN, de Kerchove d’Exaerde A, et al. Projections of nucleus accumbens adenosine A2A receptor neurons in the mouse brain and their implications in mediating sleep-wake regulation. Front Neuroanat. 2013;7:43.
Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125.
Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J Neurosci. 2012;32:15913–21.
Mogi K, Funabashi T, Mitsushima D, Hagiwara H, Kimura F. Sex difference in the response of melanin-concentrating hormone neurons in the lateral hypothalamic area to glucose, as revealed by the expression of phosphorylated cyclic adenosine 3',5'-monophosphate response element-binding protein. Endocrinology. 2005;146:3325–33.
Frankfurt M, Fuchs E, Wuttke W. Sex differences in gamma-aminobutyric acid and glutamate concentrations in discrete rat brain nuclei. Neurosci Lett. 1984;50:245–50.
Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: From electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39.
Stevenson JA, Montemurro DG. Loss of weight and metabolic rate of rats with lesions in the medial and lateral hypothalamus. Nature. 1963;198:92.
Osada T, Suzuki R, Ogawa A, Tanaka M, Hori M, Aoki S, et al. Functional subdivisions of the hypothalamus using areal parcellation and their signal changes related to glucose metabolism. Neuroimage. 2017;162:1–12.
Friend DM, Devarakonda K, O'Neal TJ, Skirzewski M, Papazoglou I, Kaplan AR, et al. Basal Ganglia Dysfunction Contributes to Physical Inactivity in Obesity. Cell Metab. 2017;25:312–21.
Michaelides M, Miller ML, Egervari G, Primeaux SD, Gomez JL, Ellis RJ et al. Striatal Rgs4 regulates feeding and susceptibility to diet-induced obesity. Mol Psychiatry. 2018. https://doi.org/10.1038/s41380-018-0120-7.
Casper RC, Sullivan EL, Tecott L. Relevance of animal models to human eating disorders and obesity. Psychopharmacology. 2008;199:313–29.
Acknowledgements
We thank Dr Walter Kaye for insightful discussions regarding this work.
Funding
SCD was supported by an IMHRO Rising Star Depression Research Award in Memory of George Largay and R21MH115395. ACW and JL were supported by R21MH115395 to SCD. JAJ was supported by R01MH54137 and CK by R01MH093672.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Welch, A.C., Zhang, J., Lyu, J. et al. Dopamine D2 receptor overexpression in the nucleus accumbens core induces robust weight loss during scheduled fasting selectively in female mice. Mol Psychiatry 26, 3765–3777 (2021). https://doi.org/10.1038/s41380-019-0633-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0633-8
This article is cited by
-
Nucleus accumbens D1- and D2-expressing neurons control the balance between feeding and activity-mediated energy expenditure
Nature Communications (2024)
-
Identification of adipose tissue transcriptomic memory of anorexia nervosa
Molecular Medicine (2023)
-
AgRP neurons coordinate the mitigation of activity-based anorexia
Molecular Psychiatry (2023)
-
How changes in dopamine D2 receptor levels alter striatal circuit function and motivation
Molecular Psychiatry (2022)
-
A D2 to D1 shift in dopaminergic inputs to midbrain 5-HT neurons causes anorexia in mice
Nature Neuroscience (2022)