Abstract
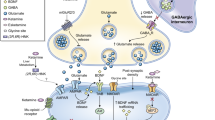
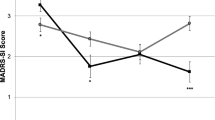
We recently reported that naltrexone blocks antidepressant effects of ketamine in humans, indicating that antidepressant effects of ketamine require opioid receptor activation. However, it is unknown if opioid receptors are also involved in ketamine’s antisuicidality effects. Here, in a secondary analysis of our recent clinical trial, we test whether naltrexone attenuates antisuicidality effects of ketamine. Participants were pretreated with naltrexone or placebo prior to intravenous ketamine in a double-blinded crossover design. Suicidality was measured with the Hamilton Depression Rating Scale item 3, Montgomery–Åsberg Depression Rating Scale item 10, and Columbia Suicide Severity Rating Scale. In the 12 participants who completed naltrexone and placebo conditions, naltrexone attenuated the antisuicidality effects of ketamine on all three suicidality scales/subscales (linear mixed model, fixed pretreatment effect, p < 0.01). Results indicate that opioid receptor activation plays a significant role in the antisuicidality effects of ketamine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Stone DM, Simon TR, Fowler KA, Kegler SR, Yuan K, Holland KM, et al. Trends in state suicide rates—United States, 1999–2016 and circumstances contributing to suicide—27 States, 2015. Morb Mortal Wkly Rep. 2018;67:617–24.
Centers for Disease Control and Prevention. Injury prevention & control: data & statistics (WISQARS). 2017. https://www.cdc.gov/injury/wisqars/. Accessed 24 Nov 2018.
Gibbons RD, Hur K, Mann J. Suicide rates and the declining psychiatric hospital bed capacity in the United States. JAMA Psychiatry. 2017;74:849–50.
Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175:620–30.
Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A. et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175:150–8.
Grunebaum MF, Galfalvy HC, Choo T-H, Keilp JG, Moitra VK, Parris MS et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2017. 10.1176/appi.ajp.2017.17060647.
Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017;19:176–83.
Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–6.
Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. 2015;45:3571–80.
Strasburger SE, Bhimani PM, Kaabe JH, Krysiak JT, Nanchanatt DL, Nguyen TN, et al. What is the mechanism of ketamine’s rapid-onset antidepressant effect? a concise overview of the surprisingly large number of possibilities. J Clin Pharm Ther. 2017;42:147–54.
Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205–15.
Lutz P-E, Mechawar N, Turecki G. Neuropathology of suicide: recent findings and future directions. Mol Psychiatry. 2017;22:1395–412.
Yovell Y, Bar G, Mashiah M, Baruch Y, Briskman I, Asherov J, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry. 2016;173:491–8.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Am Psychiatr Assoc. 2013. https://doi.org/10.1176/appi.books.9780890425596.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17.
Abdallah CG, Sanacora G, Duman RS, Krystal JH. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther. 2018;190:148–58.
Zarate CA, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–5.
Sleigh J, Harvey M, Voss L, Denny B. Ketamine—more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care. 2014;4:76–81.
Stanciu CN, Glass OM, Penders TM. Use of buprenorphine in treatment of refractory depression—a review of current literature. Asian J Psychiatry. 2017;26:94–98.
Lutz P-E, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206.
Ahmadi J, Sefidfard Jahromi M. Ultrarapid influence of buprenorphine on major dDepression in opioid-dependent patients: a double blind, randomized clinical trial. Subst Use Misuse. 2018;53:286–9.
Ionescu DF, Swee MB, Pavone KJ, Taylor N, Akeju O, Baer L, et al. Rapid and sustained reductions in current suicidal ideation following repeated doses of intravenous ketamine: secondary analysis of an open-label study. J Clin Psychiatry. 2016;77:e719–725.
Oquendo MA, Volkow ND. Suicide: a silent contributor to opioid-overdose deaths. N Engl J Med. 2018;378:1567–9.
Fornili K. The opioid crisis, suicides, and related conditions: multiple clustered syndemics, not singular epidemics. J Addict Nurs. 2018;29:214–20.
Ducasse D, Holden RR, Boyer L, Artéro S, Calati R, Guillaume S et al. Psychological pain in suicidality: a meta-analysis. J Clin Psychiatry. 2018;79:44–51.
Conejero I, Olié E, Calati R, Ducasse D, Courtet P. Psychological pain, depression, and suicide: recent evidences and future directions. Curr Psychiatry Rep. 2018;20:1–9.
Calati R, Olié E, Ritchie K, Artero S, Courtet P. Suicidal ideation and suicide attempts in the elderly associated with opioid use and pain sensitivity. Psychother Psychosom. 2017;86:373–5.
Olié E, Courtet P, Poulain V, Guillaume S, Ritchie K, Artero S. History of suicidal behaviour and analgesic use in community-dwelling elderly. Psychother Psychosom. 2013;82:341–3.
Garland EL, Riquino MR, Priddy SE, Bryan CJ. Suicidal ideation is associated with individual differences in prescription opioid craving and cue-reactivity among chronic pain patients. J Addict Dis. 2017;36:23–29.
Riquino MR, Priddy SE, Howard MO, Garland EL. Emotion dysregulation as a transdiagnostic mechanism of opioid misuse and suicidality among chronic pain patients. Borderline Personal Disord Emot Dysregulation. 2018;5:1–9.
Taylor JJ, Borckardt JJ, George MS. Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain. 2012;153:1219–25.
Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR, et al. Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS. Neuropsychopharmacology. 2013;38:1189–97.
George MS, Raman R, Benedek DM, Pelic CG, Grammer GG, Stokes KT, et al. A two-site pilot randomized 3 day trial of high dose left prefrontal repetitive transcranial magnetic stimulation (rTMS) for suicidal inpatients. Brain Stimul. 2014;7:421–31.
Weissman CR, Blumberger DM, Brown PE, Isserles M, Rajji TK, Downar J, et al. Bilateral repetitive transcranial magnetic stimulation decreases suicidal ideation in depression. J Clin Psychiatry. 2018;79:e11692.
Qin BY, Dai LL, Zheng Y. Efficacy of repetitive transcranial magnetic stimulation for alleviating clinical symptoms and suicidal ideation in elderly depressive patients: a randomized controlled trial. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37:97–101.
Scherrer JF, Salas J, Sullivan MD, Ahmedani BK, Copeland LA, Bucholz KK. et al. Impact of adherence to antidepressants on long-term prescription opioid use cessation. Br J Psychiatry. 2018;212:103–11.
Lutz P-E, Courtet P, Calati R. The opioid system and the social brain: implications for depression and suicide. J Neurosci Res. 2018;1–13. https://doi.org/10.1002/jnr.24269 [Epub ahead of print].
Lee JD, Nunes EV, Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309–18.
Schepis TS, Simoni-Wastila L, McCabe SE. Prescription opioid and benzodiazepine misuse is associated with suicidal ideation in older adults. Int J Geriatr Psychiatry. 2019;34:122–9. https://doi.org/10.1002/gps.4999.
Kuramoto SJ, Chilcoat HD, Ko J, Martins SS. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among US adults. J Stud Alcohol Drugs. 2012;73:178–84.
Ashrafioun L, Bishop TM, Conner KR, Pigeon WR. Frequency of prescription opioid misuse and suicidal ideation, planning, and attempts. J Psychiatr Res. 2017;92:1–7.
Ilgen MA, Bohnert ASB, Ganoczy D, Bair MJ, Mccarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157:1079–84.
Semenkovich K, Chockalingam R, Scherrer JF, Panagopoulos VN, Lustman PJ, Ray JM, et al. Prescription opioid analgesics increase risk of major depression: new evidence, plausible neurobiological mechanisms and management to achieve depression prophylaxis. Mo Med. 2014;111:148–54.
Salas J, Scherrer JF, Schneider FD, Sullivan MD, Bucholz KK, Burroughs T, et al. New-onset depression following stable, slow, and rapid rate of prescription opioid dose escalation. PAIN. 2017;158:306.
Salas J, Scherrer JF, Ahmedani BK, Copeland LA, Bucholz KK, Sullivan MD, et al. Gender and the association between long-term prescription opioid use and new-onset depression. J Pain. 2018;19:88–98.
Scherrer JF, Salas J, Copeland LA, Stock EM, Schneider FD, Sullivan M, et al. Increased risk of depression recurrence after initiation of prescription opioids in noncancer pain patients. J Pain. 2016;17:473–82.
Scherrer JF, Salas J, Sullivan MD, Schneider FD, Bucholz KK, Burroughs T, et al. The influence of prescription opioid use duration and dose on development of treatment resistant depression. Prev Med. 2016;91:110–6.
Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry. 2019;76:337–8. https://doi.org/10.1001/jamapsychiatry.2018.3990.
Marton T, Barnes DE, Wallace A, Woolley JD. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry. 2019;85:e75–6. https://doi.org/10.1016/j.biopsych.2019.02.008.
Heifets BD, Williams NR, Bentzley BS, Schatzberg AF. Rigorous trial design is essential to understand the role of opioid receptors in ketamine’s antidepressant effect. JAMA Psychiatry. 2019;76:657–8. https://doi.org/10.1001/jamapsychiatry.2019.0766.
Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, et al. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci USA. 2008;105:5277–81.
Copeland WE, Sun H, Costello EJ, Angold A, Heilig MA, Barr CS. Child μ-opioid receptor gene variant influences parent–child relations. Neuropsychopharmacology. 2011;36:1165–70.
Briand LA, Hilario M, Dow HC, Brodkin ES, Blendy JA, Berton O. Mouse model of OPRM1 (A118G) polymorphism increases sociability and dominance and confers resilience to social defeat. J Neurosci. 2015;35:3582–90.
Carlezon WA, Krystal AD. Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress Anxiety. 2016;33:895–906.
Browne CA, Falcon E, Robinson SA, Berton O, Lucki I. Reversal of stress-induced social interaction deficits by buprenorphine. Int J Neuropsychopharmacol. 2018;21:164–74.
Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, Kirby LG, et al. Antidepressant-like effects of buprenorphine are mediated by kappa opioid receptors. Neuropsychopharmacology. 2016;41:2344–51.
Malcolm R, O’Neil PM, Von JM, Dickerson PC. Naltrexone and dysphoria: a double-blind placebo controlled trial. Biol Psychiatry. 1987;22:710–6.
Miotto K, McCann M, Basch J, Rawson R, Ling W. Naltrexone and dysphoria: fact or myth? Am J Addict. 2002;11:151–60.
Dean AJ, Saunders JB, Jones RT, Young RM, Connor JP, Lawford BR. Does naltrexone treatment lead to depression? Findings from a randomized controlled trial in subjects with opioid dependence. J Psychiatry Neurosci. 2006;31:38–45.
Carroll KM, Nich C, Frankforter TL, Yip SW, Kiluk BD, DeVito EE, et al. Accounting for the uncounted: physical and affective distress in individuals dropping out of oral naltrexone treatment for opioid use disorder. Drug Alcohol Depend. 2018;192:264–70.
Kelty E, Hulse G. Fatal and non-fatal opioid overdose in opioid dependent patients treated with methadone, buprenorphine or implant naltrexone. Int J Drug Policy. 2017;46:54–60.
Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107:1817–24.
Mischoulon D, Hylek L, Yeung AS, Clain AJ, Baer L, Cusin C, et al. Randomized, proof-of-concept trial of low dose naltrexone for patients with breakthrough symptoms of major depressive disorder on antidepressants. J Affect Disord. 2017;208:6–14.
Murphy BL, Ravichandran C, Babb SM, Cohen BM. Naltrexone in bipolar disorder with depression: a double-blind, placebo-controlled study. J Clin Psychopharmacol. 2014;34:749–51.
Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, et al. A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry. 2010;167:668–75.
Funding
This work was supported by the Stanford Clinical and Translational Science Award to Spectrum [NIH UL1 TR 001085] (NRW + AFS); the 2016 and 2018 NARSAD Young Investigator Grant program (NRW); the Avy L. and Robert L. Miller Foundation (NRW); NIMH K08MH110610 (BDH) and the Pritzker Family Fund (AFS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
AFS reports Consulting: Bracket/Clintara; Alkermes; Neuronetics; McKinsey; GLG Consulting; Avanir. Equity: Xhale; Corcept; Merck; Seattle Genetics; Gilead; Titan; Incyte Genetics; Intersect. Grants; Janssen. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Williams, N.R., Heifets, B.D., Bentzley, B.S. et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry 24, 1779–1786 (2019). https://doi.org/10.1038/s41380-019-0503-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0503-4
This article is cited by
-
Progesterone receptor distribution in the human hypothalamus and its association with suicide
Acta Neuropathologica Communications (2024)
-
Sex dependence of opioid-mediated responses to subanesthetic ketamine in rats
Nature Communications (2024)
-
The endogenous opioid system in the medial prefrontal cortex mediates ketamine’s antidepressant-like actions
Translational Psychiatry (2024)
-
The why, when, where, how, and so what of so-called rapidly acting antidepressants
Neuropsychopharmacology (2024)
-
Mechanism of Antidepressant Action of (2R,6R)-6-Hydroxynorketamine (HNK) and Its Compounds: Insights from Proteomic Analysis
Molecular Neurobiology (2024)