Abstract
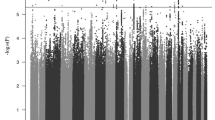
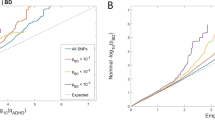
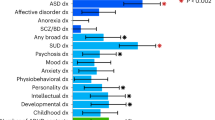
Attention-deficit/hyperactivity disorder (ADHD) is a severely impairing neurodevelopmental disorder with a prevalence of 5% in children and adolescents and of 2.5% in adults. Comorbid conditions in ADHD play a key role in symptom progression, disorder course and outcome. ADHD is associated with a significantly increased risk for substance use, abuse and dependence. ADHD and cannabis use are partly determined by genetic factors; the heritability of ADHD is estimated at 70–80% and of cannabis use initiation at 40–48%. In this study, we used summary statistics from the largest available meta-analyses of genome-wide association studies (GWAS) of ADHD (n = 53,293) and lifetime cannabis use (n = 32,330) to gain insights into the genetic overlap and causal relationship of these two traits. We estimated their genetic correlation to be r2 = 0.29 (P = 1.63 × 10−5) and identified four new genome-wide significant loci in a cross-trait analysis: two in a single variant association analysis (rs145108385, P = 3.30 × 10−8 and rs4259397, P = 4.52 × 10−8) and two in a gene-based association analysis (WDPCP, P = 9.67 × 10−7 and ZNF251, P = 1.62 × 10−6). Using a two-sample Mendelian randomization approach we found support that ADHD is causal for lifetime cannabis use, with an odds ratio of 7.9 for cannabis use in individuals with ADHD in comparison to individuals without ADHD (95% CI (3.72, 15.51), P = 5.88 × 10−5). These results substantiate the temporal relationship between ADHD and future cannabis use and reinforce the need to consider substance misuse in the context of ADHD in clinical interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
25 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41380-021-01049-6
References
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–8.
Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194:204–11.
Du Rietz E, Kuja-Halkola R, Brikell I, Jangmo A, Sariaslan A, Lichtenstein P, et al. Predictive validity of parent- and self-rated ADHD symptoms in adolescence on adverse socioeconomic and health outcomes. Eur Child Adolesc Psychiatry. 2017;26:857–67.
Cuffe SP, Visser SN, Holbrook JR, Danielson ML, Geryk LL, Wolraich ML, et al. ADHD and psychiatric comorbidity: functional outcomes in a school-based sample of children. J Atten Disord. 2015. Epub ahead of print; https://doi.org/10.1177/1087054715613437.
Franke B, Michelini G, Asherson P, Banaschewski T, Bilbow A, Buitelaar JK, et al. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur Neuropsychopharmacol. 2018;28:1059–1088.
Taurines R, Schmitt J, Renner T, Conner AC, Warnke A, Romanos M. Developmental comorbidity in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2010;2:267–89.
Jacob CP, Romanos J, Dempfle A, Heine M, Windemuth-Kieselbach C, Kruse A, et al. Co-morbidity of adult attention-deficit/hyperactivity disorder with focus on personality traits and related disorders in a tertiary referral center. Eur Arch Psychiatry Clin Neurosci. 2007;257:309–17.
Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31:328–41.
McGough JJ, Smalley SL, McCracken JT, Yang M, Del’Homme M, Lynn DE, et al. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: findings from multiplex families. Am J Psychiatry. 2005;162:1621–7.
Biederman J, Wilens T, Mick E, Milberger S, Spencer TJ, Faraone SV. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;152:1652–8.
Mannuzza S, Klein RG, Moulton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160:237–46.
Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann N Y Acad Sci. 2001;931:251–70.
Molina BS, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52:250–63.
Fergusson DM, Boden JM. Cannabis use and adult ADHD symptoms. Drug Alcohol Depend. 2008;95:90–6.
Estevez N, Dey M, Eich-Hochli D, Foster S, Gmel G, Mohler-Kuo M. Adult attention-deficit/hyperactivity disorder and its association with substance use and substance use disorders in young men. Epidemiol Psychiatr Sci. 2016;25:255–66.
De Alwis D, Agrawal A, Reiersen AM, Constantino JN, Henders A, Martin NG, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs. 2014;75:211–21.
Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50:9–21.
Sibley MH, Pelham WE, Molina BSG, Coxe S, Kipp H, Gnagy EM, et al. The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. J Abnorm Psychol. 2014;123:362–74.
Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry. 2007;64:1145–52.
Loflin M, Earleywine M, De Leo J, Hobkirk A. Subtypes of attention deficit-hyperactivity disorder (ADHD) and cannabis use. Subst Use Misuse. 2014;49:427–34.
Pingault JB, Cote SM, Galera C, Genolini C, Falissard B, Vitaro F, et al. Childhood trajectories of inattention, hyperactivity and oppositional behaviors and prediction of substance abuse/dependence: a 15-year longitudinal population-based study. Mol Psychiatry. 2013;18:806–12.
Agnew-Blais JC, Polanczyk GV, Danese A, Wertz J, Moffitt TE, Arseneault L. Evaluation of the persistence, remission, and emergence of attention-deficit/hyperactivity disorder in young adulthood. JAMA Psychiatry. 2016;73:713–20.
Larsson H, Chang Z, D’Onofrio BM, Lichtenstein P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol Med. 2014;44:2223–9.
Franke B, Faraone SV, Asherson P, Buitelaar J, Bau CH, Ramos-Quiroga JA, et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry. 2012;17:960–87.
Asherson P, Gurling H. Quantitative and molecular genetics of ADHD. Curr Top Behav Neurosci. 2012;9:239–72.
Faraone SV, Larsson HL. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2018. Epub ahead of print; https://doi.org/10.1038/s41380-018-0070-0.
Verweij KJ, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010;105:417–30.
Turner S (2017). qqman: Q-Q and Manhattan Plots for GWAS Data. R package version 0.1.4. http://CRANR-projectorg/package=qqman.
Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for ADHD. bioRxiv. 101101/145581. 2017.
Stringer S, Minica CC, Verweij KJ, Mbarek H, Bernard M, Derringer J, et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl Psychiatry. 2016;6:e769.
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65.
Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–41.
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7.
Yang J, Ferreira T, Morris AP, Medland SE, Madden PA, Heath AC, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44(S1-3):369–75. (4)
de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol. 2015;11:e1004219.
Hou C-D. A simple approximation for the distribution of the weighted combination of non-independent or independent probabilities. Stat & Probab Lett. 2005;73:179–87.
Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–7.
R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-projectorg/.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Gibran Hemani PHaJZ. TwoSampleMR: two sample MR functions and interface to MR Base database. R package version 030.
Olena Yavorska (2017). MendelianRandomization: Mendelian Randomization Package. R package version 0.2.2. https://CRANR-projectorg/package=MendelianRandomization.
Vaucher J, Keating BJ, Lasserre AM, Gan W, Lyall DM, Ward J, et al. Cannabis use and risk of schizophrenia: a Mendelian randomization study. Mol Psychiatry. 2018;23:1287–1292.
Ross S, Gerstein HC, Eikelboom J, Anand SS, Yusuf S, Pare G. Mendelian randomization analysis supports the causal role of dysglycaemia and diabetes in the risk of coronary artery disease. Eur Heart J. 2015;36:1454–62.
Groenman AP, Greven CU, van Donkelaar MM, Schellekens A, van Hulzen KJ, Rommelse N, et al. Dopamine and serotonin genetic risk scores predicting substance and nicotine use in attention deficit/hyperactivity disorder. Addict Biol. 2016 Jul;21:915–23.
Arcos-Burgos M, Velez JI, Solomon BD, Muenke M. A common genetic network underlies substance use disorders and disruptive or externalizing disorders. Hum Genet. 2012;131:917–29.
van Hulzen KJE, Scholz CJ, Franke B, Ripke S, Klein M, McQuillin A, et al. Genetic overlap between attention-deficit/hyperactivity disorder and bipolar disorder: evidence from genome-wide association study meta-analysis. Biol Psychiatry. 2017;82:634–41.
Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, et al. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry. 2016;21:1485.
McKay JD, Hung RJ, Han Y, Zong X, Carreras-Torres R, Christiani DC, et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet. 2017;49:1126–32.
Kumar R, Cheney KM, McKirdy R, Neilsen PM, Schulz RB, Lee J, et al. CBFA2T3-ZNF652 corepressor complex regulates transcription of the E-box gene HEB. J Biol Chem. 2008;283:19026–38.
GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5.
Aebi M, van Donkelaar MM, Poelmans G, Buitelaar JK, Sonuga-Barke EJ, Stringaris A, et al. Gene-set and multivariate genome-wide association analysis of oppositional defiant behavior subtypes in attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2016;171:573–88.
Arloth J, Bogdan R, Weber P, Frishman G, Menke A, Wagner KV, et al. Genetic differences in the immediate transcriptome response to stress predict risk-related brain function and psychiatric disorders. Neuron. 2015;86:1189–202.
Krumm N, O’Roak BJ, Karakoc E, Mohajeri K, Nelson B, Vives L, et al. Transmission disequilibrium of small CNVs in simplex autism. Am J Hum Genet. 2013;93:595–606.
Spiers H, Hannon E, Schalkwyk LC, Smith R, Wong CC, O’Donovan MC, et al. Methylomic trajectories across human fetal brain development. Genome Res. 2015;25:338–52.
Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB, et al. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1345–54.
Zhang-James Y, DasBanerjee T, Sagvolden T, Middleton FA, Faraone SV. SLC9A9 mutations, gene expression, and protein-protein interactions in rat models of attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:835–43.
Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, et al. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify “connectivity constellation” and drug target genes with pleiotropic effects. Ann N Y Acad Sci. 2008;1141:318–81.
Tsui D, Vessey JP, Tomita H, Kaplan DR, Miller FD. FoxP2 regulates neurogenesis during embryonic cortical development. J Neurosci. 2013;33:244–58.
Uhl GR, Drgon T, Johnson C, Fatusin OO, Liu QR, Contoreggi C, et al. “Higher order” addiction molecular genetics: convergent data from genome-wide association in humans and mice. Biochem Pharmacol. 2008;75:98–111.
Drgon T, Johnson CA, Nino M, Drgonova J, Walther DM, Uhl GR. “Replicated” genome wide association for dependence on illegal substances: genomic regions identified by overlapping clusters of nominally positive SNPs. Am J Med Genet B Neuropsychiatr Genet. 2011;156:125–38.
Johnson C, Drgon T, Liu QR, Zhang PW, Walther D, Li CY, et al. Genome wide association for substance dependence: convergent results from epidemiologic and research volunteer samples. BMC Med Genet. 2008;9:113.
Drgonova J, Walther D, Wang KJ, Hartstein GL, Lochte B, Troncoso J, et al. Mouse model for PTPRD associations with WED/RLS and addiction: reduced expression alters locomotion, sleep behaviors and cocaine-conditioned place preference. Mol Med. 2015;21:717–725.
Drgon T, Zhang PW, Johnson C, Walther D, Hess J, Nino M, et al. Genome wide association for addiction: replicated results and comparisons of two analytic approaches. PLoS ONE. 2010;5:e8832.
Davis C, Cohen A, Davids M, Rabindranath A. Attention-deficit/hyperactivity disorder in relation to addictive behaviors: a moderated-mediation analysis of personality-risk factors and sex. Front Psychiatry. 2015;6:47.
Bidwell LC, Henry EA, Willcutt EG, Kinnear MK, Ito TA. Childhood and current ADHD symptom dimensions are associated with more severe cannabis outcomes in college students. Drug Alcohol Depend. 2014;135:88–94.
De Alwis D, Lynskey MT, Reiersen AM, Agrawal A. Attention-deficit/hyperactivity disorder subtypes and substance use and use disorders in NESARC. Addict Behav. 2014;39:1278–85.
Acknowledgements
We are grateful to patients from the Hospital Universitari Vall d’Hebron who kindly participated in this research. Genotyping was performed at the Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, Massachusetts, United States of America. Statistical analyses were carried out on the Genetic Cluster Computer (http://www.geneticcluster.org) hosted by SURFsara and financially supported by the Netherlands Scientific Organization (NWO 480-05-003 PI: Posthuma) along with a supplement from the Dutch Brain Foundation and the VU University Amsterdam. S.V.F. is supported by the K.G. Jebsen Center for Research on Neuropsychiatric Disorders, University of Bergen, Bergen, Norway, the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no 602805, the European Union’s Horizon 2020 research and innovation programme under grant agreements No 667302 & 728018 and NIMH grants 5R01MH101519 and U01 MH109536-01. B.F.‘s research is supported by a personal grant from the Netherlands Organization for Scientific Research (NWO) Vici Innovation Program (grant 016-130-669). Additional support is received from the European Community’s Seventh Framework Programme (FP7/2007-13) under grant agreement n° 602805 (Aggressotype), and from the European Community’s Horizon 2020 Programme (H2020/2014-20) under grant agreements n° 643051 (MiND), and n° 667302 (CoCA). I.G.M. is a recipient of a contract from the 7th Framework Programme for Research, Technological Development and Demonstration, European Commission (AGGRESSOTYPE_FP7HEALTH2013/602805). B.M.N. was supported by the National Institutes of Health (1R01MH094469). Over the course of this investigation, M.P. has been a recipient of a pre-doctoral fellowship from the Vall d’Hebron Research Institute (PRED-VHIR-2013) and a research grant from the Deutscher Akademischer Austauschdienst (DAAD), Germany (Research Grants - Short-Term Grants, 2017). M.R. is a recipient of a Miguel de Servet contract from the Instituto de Salud Carlos III, Spain (CP09/00119 and CPII15/00023). P.R. is a recipient of a pre-doctoral fellowship from the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR), Generalitat de Catalunya, Spain (2016FI_B 00899). C.S.M. is a recipient of a Sara Borrell contract and a mobility grant from the Spanish Ministerio de Economía y Competitividad, Instituto de Salud Carlos III (CD15/00199 and MV16/00039). M.S.A. is a recipient of a contract from the Biomedical Network Research Center on Mental Health (CIBERSAM), Madrid, Spain. J.M.V. was supported by the European Research Council (ERC-284167). This work was funded by Instituto de Salud Carlos III (PI14/01700, PI15/01789, PI16/01505, PI17/00289), and co-financed by the European Regional Development Fund (ERDF), Agència de Gestió d’Ajuts Universitaris i de Recerca-AGAUR, Generalitat de Catalunya, Spain (2014SGR1357, 2017SGR1461), the Health Research and Innovation Strategy Plan (PERIS SLT006/17/287), Generalitat de Catalunya, Spain, the European College of Neuropsychopharmacology (ECNP network: ‘ADHD across the lifespan’), Departament de Salut, Generalitat de Catalunya, Spain, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. The research leading to these results has received funding from the European Union Seventh Framework Program (FP72007-2013) under grant agreement No 602805 and from the European Union H2020 Programme (H2020/2014-20) under grant agreements Nos. 667302 (CoCA) and 643051 (MiND) and 728018 (Eat2BeNICE). The iPSYCH project is funded by the Lundbeck Foundation (grant numbers R102-A9118 and R155-2014-1724) and the universities and university hospitals of Aarhus and Copenhagen. The European Community’s Horizon 2020 Programme (H2020/2014-20) under Grant No. 667302 (CoCA). Analyses of the iPSYCH data was done using the high-performance computer capacity on the GenomeDK HPC facility provided by the Center for Integrative Sequencing, iSEQ, Aarhus Genome Center, Aarhus University, Denmark, funded by grants from Aarhus University and Aarhus University Hospital.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest
M.C. has received travel grants and research support from Eli Lilly and Co., Janssen-Cilag, Shire, and Laboratorios Rubió. He has been on the advisory board and served as a consultant for Eli Lilly and Co., Janssen-Cilag, Shire, and Laboratorios Rubió. In the past year, S.V.F. received income, potential income, travel expenses continuing education support and/or research support from Otsuka, Arbor, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA, Ironshore, Sunovion, and Genomind. With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. B.F. has received educational speaking fees from Shire and Medice. J.A.R.Q. has served on the speakers’ bureau and acted as a consultant for Eli Lilly and Co., Janssen-Cilag, Novartis, Lundbeck, Shire, Ferrer, and Laboratorios Rubió. He has received travel awards from Eli Lilly and Co., Janssen-Cilag, and Shire for participating in psychiatric meetings. The ADHD Program chaired by J.A.R.Q. has received unrestricted educational and research support from Eli Lilly and Co., Janssen-Cilag, Shire, Rovi, and Laboratorios Rubió in the past two years. B.N. is a member of the scientific advisory board at Deep Genomics and RBNC Therapeutics and a consultant for Camp4 Therapeutics, Takeda Pharmaceutical and Biogen. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Following publication of this article, Benjamin M. Neale contacted the journal to request the addition of the following text to the ‘Conflict of Interest’ declaration: ‘B.N. is a member of the scientific advisory board at Deep Genomics and RBNC Therapeutics and a consultant for Camp4 Therapeutics, Takeda Pharmaceutical and Biogen’.
Supplementary information
Rights and permissions
About this article
Cite this article
Soler Artigas, M., Sánchez-Mora, C., Rovira, P. et al. Attention-deficit/hyperactivity disorder and lifetime cannabis use: genetic overlap and causality. Mol Psychiatry 25, 2493–2503 (2020). https://doi.org/10.1038/s41380-018-0339-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0339-3
This article is cited by
-
The impact of recreational cannabis legalization on youth: the Colorado experience
European Child & Adolescent Psychiatry (2024)
-
Genome-wide association study of population-standardised cognitive performance phenotypes in a rural South African community
Communications Biology (2023)
-
An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder
Discover Mental Health (2023)
-
The genetic aetiology of cannabis use: from twin models to genome-wide association studies and beyond
Translational Psychiatry (2022)
-
Addictive and other mental disorders: a call for a standardized definition of dual disorders
Translational Psychiatry (2022)