Abstract
Categorizing people with late-onset Alzheimer’s disease into biologically coherent subgroups is important for personalized medicine. We evaluated data from five studies (total n = 4050, of whom 2431 had genome-wide single-nucleotide polymorphism (SNP) data). We assigned people to cognitively defined subgroups on the basis of relative performance in memory, executive functioning, visuospatial functioning, and language at the time of Alzheimer’s disease diagnosis. We compared genotype frequencies for each subgroup to those from cognitively normal elderly controls. We focused on APOE and on SNPs with p < 10−5 and odds ratios more extreme than those previously reported for Alzheimer’s disease (<0.77 or >1.30). There was substantial variation across studies in the proportions of people in each subgroup. In each study, higher proportions of people with isolated substantial relative memory impairment had ≥1 APOE ε4 allele than any other subgroup (overall p = 1.5 × 10−27). Across subgroups, there were 33 novel suggestive loci across the genome with p < 10−5 and an extreme OR compared to controls, of which none had statistical evidence of heterogeneity and 30 had ORs in the same direction across all datasets. These data support the biological coherence of cognitively defined subgroups and nominate novel genetic loci.
Similar content being viewed by others
Introduction
Clinical heterogeneity is common among people with late-onset Alzheimer’s disease (see [1] for a review). Categorizing people with a condition into biologically coherent subgroups is an important personalized medicine strategy [2]. This strategy is particularly recommended for neurodegenerative conditions [3]. Once biologically coherent subgroups are identified, further investigations may elucidate subgroup-specific treatments.
Genetic data may be useful for determining whether a proposed categorization strategy results in biologically coherent subgroups (see the Box 1).
We have developed an approach for categorizing people with late-onset Alzheimer’s disease based on relative performance across cognitive domains. We determine each person’s average performance at diagnosis across memory, executive functioning, language, and visuospatial ability, and consider relative impairments in each domain from that average. We previously evaluated one study’s data and showed that our strategy identified a subgroup with higher degrees of amyloid angiopathy and higher proportions with≥1 APOE ε4 allele [4].
Here, we evaluate data from five studies with people with late-onset Alzheimer’s disease [5] and cognitively normal elderly controls [6]. We used modern psychometric approaches to co-calibrate cognitive scores. We used scores to identify subgroups. We used genetic data to determine whether our categorization identifies biologically coherent subgroups.
Materials and methods
Study design and participants
We used data from the Adult Changes in Thought (ACT) study, the Alzheimer’s Disease Neuroimaging Initiative (ADNI), the Rush Memory and Aging Project (MAP) and Religious Orders Study (ROS), and the University of Pittsburgh Alzheimer Disease Research Center (PITT). Each study has published widely, and their genetic data are included in large analyses of late-onset Alzheimer’s disease [6, 7]. All five studies use the same research criteria to define clinical Alzheimer’s disease [5]. Three studies (ACT, MAP, and ROS) are prospective cohort studies that enroll cognitively normal elderly individuals and follow them to identify incident dementia cases. For these, we analyzed cognitive data from the visit with the incident clinical Alzheimer’s disease diagnosis. Two studies (ADNI and PITT) are clinic-based research cohort studies. For those studies we analyzed cognitive data from the first study visit for people with prevalent Alzheimer’s disease; we limited inclusion to those with Clinical Dementia Rating Scale [8] of 0.5 or 1, indicating mild Alzheimer’s disease. For people from ADNI or PITT who did not initially have Alzheimer’s disease, we analyzed cognitive data from the incident Alzheimer’s disease visit. The University of Washington IRB approved these secondary analyses of coded data.
In each study, we included people diagnosed with late-onset Alzheimer’s disease as defined by research criteria [5]. We used data from everyone for all analyses other than genetic analyses; we limited those to self-reported whites. For genetic analyses we also used data from self-reported white cognitively normal elderly controls from each study. Details on those cohorts are included in reports from the parent studies and in Supplementary Text 6 (derived from Lambert et al. [6]).
Cognitive data procedures
Staff from each study administered a comprehensive neuropsychological test battery that included assessment of memory, executive functioning, language, and visuospatial functioning. We obtained granular (“item-level”) data from each parent study. Each stimulus administered to a participant was deemed an “item”. As outlined in our previous paper [2], every item administered in each study was considered by our panel of experts (JM, ET, AJS). Our panel designated each item as primarily a measure of memory, executive functioning, language, visuospatial functioning, or none of these. They also assigned items to theory-driven subdomains.
We carefully considered items where the same stimulus was administered to participants across different studies to identify “anchor items” that could anchor metrics across studies. We reviewed response categories recorded by each study for these overlapping items to ensure that consistent scoring was used across studies. We identified anchor items as those overlapping items with identical scoring across studies. We used bifactor models in Mplus [9] to co-calibrate separate scores for memory, executive functioning, language, and visuospatial functioning.
Details of item assignments, psychometric analyses in each study, and co-calibration methodology across studies are provided in Supplementary Texts 1, 2, and 5. Code is available from the authors upon request.
We used the ACT sample of people with incident Alzheimer’s disease as our reference population for the purpose of scaling domain scores; ACT was our largest sample from a prospective cohort study of people with late-onset Alzheimer’s disease. We z-transformed scores from other studies to ACT-defined metrics for each domain.
Assignment to subgroups
We assigned people to subgroups as we have done previously [4]. Briefly, for each person we determined their average score across memory, executive functioning, language, and visuospatial functioning. We determined differences between each domain score and this average score. We identified domains with substantial relative impairments as those with relative impairments at least as large as 0.80 standard deviation units as explained in Supplementary Text 3. We categorized people by the number of domains with substantial relative impairments (0, 1, or ≥2) and further categorized those with substantial relative impairments in a single domain by the domain with a substantial relative impairment. This approach results in six potential subgroups: those with no domain with a substantial relative impairment; those with an isolated substantial relative impairment in one of four domains (e.g., isolated substantial relative memory impairment, isolated substantial relative language impairment, etc.), and those with multiple domains with substantial relative impairments.
Statistical analyses
As in our previous study [4], we compared the proportion of people with late-onset Alzheimer’s disease in each subgroup with at least one APOE ε4 allele. For other genetic analyses we combined data from ROS and MAP, as has been done many times previously, and evaluated data separately in four genetic datasets. Each dataset was imputed using IMPUTE2 and samples of European ancestry from the 2012 build of the 1000 Genomes project. We excluded SNPs with R2 or information scores < 0.5, and SNPs with a minor allele frequency <3%. Further details are provided in Supplementary Text 6 and in Lambert et al. [6]
We used KING-Robust [10] to obtain study-specific principal components to account for population stratification. We used logistic regression in PLINK v 1.9 [11] to evaluate associations at each SNP for each cognitively defined subgroup. Cognitively normal elderly controls from each study were the comparison group for all of these analyses. We included covariates for age, sex, and principal components. We used METAL [12] for meta-analysis.
IGAP’s most extreme odds ratio (OR) outside of chromosome 19 was for rs11218343 associated with SORL1, which had an OR of 0.77 reported in the Stages 1 and 2 meta-analysis [6]. We focused attention on SNPs where meta-analysis ORs for any cognitively defined subgroup were < 0.77 (or, equivalently, ORs > [1/0.77], which is >1.30). As presented in the Box 1, more extreme ORs in a single subgroup, with strong replication across genetic datasets, would represent strong support of biologically coherent categorization.
For genetic loci previously identified as associated with risk of late-onset Alzheimer’s disease, we used the methods described in ref. [13] applied to IGAP’s previously reported ORs and confidence intervals to determine significance of subgroup associations compared to IGAP.
We used genetic data from all cognitively normal elderly controls and all people with Alzheimer’s disease to generate Alzheimer’s disease and subgroup genetic risk scores. We used (1) IGAP SNPs and effect sizes to generate Alzheimer’s disease risk scores and (2) our results to generate risk scores for each of the five subgroups.
To evaluate these risk scores, we used logistic regression with Alzheimer’s disease case vs. control status as the outcome, and included terms for age and sex. We compared a model with just the addition of the IGAP genetic risk score to a model that incorporated that score plus the five subgroup risk scores. Finally, we compared area under the receiver operator characteristic (ROC) curves for the model with the IGAP risk score to a model that did not include that term but included terms for the five subgroup risk scores. Further details are shown in Supplementary Text 12.
Data sharing
Co-calibrated scores for each domain are available from the parent studies. GWAS meta-analysis summary statistics for each subgroup will be available on the National Institute on Aging Genetics of Alzheimer’s Disease Storage Site (NIAGADS; https://www.niagads.org/datasets/ng00073). Other study materials are available here: https://digital.lib.washington.edu/researchworks/handle/1773/40364.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit the publication.
Results
In all, there were 4050 people with sufficient cognitive data to be classified into a subgroup. Demographic characteristics and average cognitive domain scores by study are shown in Table 1. Participants in the prospective cohort studies (ACT, MAP, and ROS) were older on average than those from ADNI and PITT. Most participants in each study self-reported white race (90% in ACT to 96% in MAP). There was some variation in cognitive performance across studies. The most notable differences from ACT (our reference for scaling) were for executive functioning in ADNI and ROS (average 0.8 units higher), and for language in MAP (average 0.8 units lower).
Proportions of people in each subgroup are shown in Fig. 1. There was considerable heterogeneity in proportions across studies (χ2df=20 = 468.7, p = 1.0 × 10−86).
Demographic characteristics of people in each subgroup were similar to those for people with Alzheimer’s disease overall (Supplementary Table 29). The proportion who were female ranged from 51% for isolated substantial relative executive functioning impairment to 63% of those with isolated substantial relative visuospatial impairment. Mean age at diagnosis ranged from 79 for those with isolated substantial relative visuospatial or memory impairment to 82 for those with isolated substantial relative language impairment. Mean years of education did not vary substantially across subgroups.
There were 3701 people with APOE genotype data (Table 2). We published APOE results from ACT [4]; the proportion of those with isolated substantial memory impairment with ≥ 1 APOE ε4 allele was 12% higher than overall in that study. This finding was consistent across all five studies. Overall, the proportion of people with ≥ 1 APOE ε4 allele was 15% higher in those with isolated substantial memory impairment (65%) compared with the entire sample (50%). The differences in proportions with ≥ 1 APOE ε4 allele were highly significant (p = 1.5 × 10−27). The APOE result was not sensitive to choosing other thresholds to indicate a substantial relative impairment (Supplementary Text 4 and Supplementary Fig. 2).
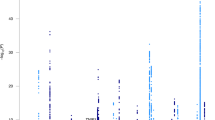
There were 2431 people with late-onset Alzheimer’s disease and 3447 cognitively normal elderly controls with genome-wide SNP data. Top results are shown in Fig. 2. There were 33 loci outside Chromosome 19 where the p-value for one subgroup was < 5 × 10−5. All of these had ORs < 0.77 or > 1.30 compared to cognitively normal elderly controls. These included nine loci for those with isolated substantial visuospatial impairment (red dots, including rs2289506 near NIT2 on chromosome 3, rs9369477 near SPATS1 on chromosome 4, rs2046197 near CSMD1 on chromosome 8, and rs8091629 near SLC14A2 on chromosome 18), nine for those with multiple domains with substantial relative impairments (yellow dots, including rs698842 near NRXN1 on chromosome 2, rs78872508 near HDAC9 on chromosome 7, and rs4348488 near BMP1 on chromosome 8), seven for those with no domain with a substantial relative impairment (purple dots, including rs11708767 near MED12L on chromosome 3, rs72839770 near DVL2 on chromosome 17, and rs7264688 near MGME1 on chromosome 20), six for those with isolated substantial language impairment (green dots, including rs13374908 near FAM163A on chromosome 1, rs28715896 near ERBB4 on chromosome 2, and rs75337321 near CACNA2D3 on chromosome 3), and two for those with isolated substantial memory impairment (blue dots, including rs1977412 near AGT on chromosome 1), and one for those with no domains with substantial impairments (purple dot, rs7264688 near MGME1 on chromosome 20). Other loci shown in Fig. 2 not named in the preceding sentence were > 50 kb from genes. Replication results are in Supplementary Tables 2a and 2b. All ORs were in the same direction for all these SNPs except rs28715896 on chromosome 2 near ERBB4, rs61835453 on chromosome 10, and rs365521 on chromosome 17. Heterogeneity p values did not suggest heterogeneity for any of these SNPs. No SNP outside the APOE region reached p < 5 × 10−8, the traditional level of genome-wide significance (Fig. 2).
Novel SNPs associated with cognitively defined subdomains with p < 10–5 and OR < 0.77 or > 1.33. CAF=coded allele frequency. SNPs further than 50 kB from a gene do not have a gene name reported here. Gray shading in the odds ratios column of the figure delineates ORs > 0.77 and < 1.30, which is the range of ORs outside APOE from the International Genomics of Alzheimer’s Project (IGAP) [6]
Results for selected IGAP loci are shown in Supplementary Table 30. We selected subgroups with meta-analytic ORs < 0.77 or > 1.30 and for which results from all four data sets were in the same direction. Results for other IGAP SNPs for all studies and all subgroups are in Supplementary Table 31.
Across all datasets, there were 5878 people with SNP data who were either normal controls or Alzheimer’s disease cases. The logistic regression model for case vs. control status with age, sex, and IGAP gene scores had a likelihood of 176.13 and a pseudo-R2 of 0.022. A model that included all of those terms and also included five subgroup gene scores had a likelihood of 311.94 and a pseudo-R2 of 0.039. These were nested models; we compared them with a likelihood ratio test with five degrees of freedom. The difference in likelihoods was highly significant (p = 1.39 × 10−27).
The area under the ROC curve for the model with age, sex, and the IGAP genetic risk score was 0.60 (95% CI 0.58, 0.61), while for the model with age, sex, and five subgroup genetic risk scores, the area under the ROC curve was 0.62 (95% CI 0.61, 0.64). This difference was statistically significant (χ2df=1 = 11.15, p = 0.0008). Further analyses are described in Supplementary Text 12.
Discussion
We used modern psychometric methods to co-calibrate cognitive data to generate domain scores across five different studies of older adults with research-quality clinical Alzheimer’s disease diagnoses. We obtained scores on the same metric, so scores from different studies were directly comparable to each other. The proportion of people with Alzheimer’s disease in each study categorized in each subgroup varied across studies (Fig. 1). We used genetic data to determine whether our categorization scheme resulted in biologically coherent subgroups. Top genetic associations from each subgroup were consistent across studies, suggesting our findings were not due to idiosyncrasies of any particular study. Gene scores for subgroups performed better in predicting case vs. control status than gene scores derived from Alzheimer’s disease case-control analyses.
APOE genotype was significantly different across subgroups. We previously showed in ACT that more people with isolated substantial relative memory impairment had at least one APOE ε4 allele than people in other subgroups [4]. We robustly replicated that finding here (Table 2). Associations between APOE ε4 alleles and memory impairment among people with Alzheimer’s disease have been previously noted [14].
Outside of chromosome 19 which was dominated by APOE-related signals for all subgroups, we identified 33 loci with p < 10−5 for at least one subgroup. All of these had ORs < 0.77 or > 1.30 (Fig. 2). There were consistent findings across datasets for nearly all these loci (Supplementary Text 7–11).
Gene scores from IGAP SNPs explained a modest amount of risk for case-control status. Adding gene scores from our cognitively defined subgroups improved prediction of Alzheimer’s disease. Furthermore, in a head-to-head comparison, gene scores for cognitively defined subgroups did a better job predicting Alzheimer’s disease status than did gene scores from IGAP SNPs.
These data provide strong support for the biological coherence of subgroups produced by our categorization scheme. Each subgroup we analyzed has extreme ORs at novel SNPs that were consistent across multiple independent samples. Even with the relatively small sample sizes from these studies, the large effect sizes at common SNPs produced p values that are close to genome-wide significance.
Others have used different data sources to categorize people with Alzheimer’s disease. Sweet and colleagues compared people with Alzheimer’s disease who developed psychosis to those who did not [15]. They identified a few interesting loci, but effect sizes were much smaller than those reported here (Supplementary Text 13).
We were part of a consortium evaluating rates of decline among people with Alzheimer’s disease [16]. The evidence in support of rates of decline, as an organizing characteristic among people with Alzheimer’s disease, is not nearly as strong as that shown here for cognitively defined subgroups.
Others have used cluster analysis approaches applied to neuropsychological [17, 18] or imaging [19, 20] data to categorize people with Alzheimer’s disease. There are very important distinctions between those approaches and the approach adopted here. In cluster analysis, the computer maximizes some distance across groups in a way that may not make clinical or biological sense. Disease severity is an important consideration (see [21] for a better discussion).
Our approach began with theory and focused exclusively on cognitive data. An early paper considered differences between memory and executive functioning among people with Alzheimer’s disease [22]. Differences between these scores enables memory to serve as something of a proxy for disease severity. This framework is useful for considering dysexecutive Alzheimer’s disease [23,24,25,26].
We have extended that framework to incorporate additional cognitive domains. The field has increasingly emphasized the importance of Alzheimer’s disease variants including primary progressive aphasia (PPA) and posterior cortical atrophy (PCA) [27]; these rare subtypes are described as typically having early onset. Clinical descriptions of the cognitive patterns of these variants emphasize relative deficits between language (PPA) or visuospatial functioning (PCA) and other domains. We thus incorporate average performance across domains, and differences from that average, to more fully capture the range of clinical heterogeneity described in late onset Alzheimer’s disease [1].
Our investigation of cognitively defined late-onset Alzheimer’s disease was motivated by considerations of previously described Alzheimer’s disease variants, including logopenic PPA and PCA. Overlap between logopenic PPA and a history of dyslexia has led to the question of whether altered patterns of language network connectivity associated with dyslexia may predispose to selective regional vulnerability to Alzheimer’s pathological processes [28]. The work described here focuses exclusively on people with typical late-onset Alzheimer’s disease and demonstrates substantial numbers of people with patterns of relative cognitive impairment that are similar to those described in rare Alzheimer’s subtypes, such as logopenic PPA and PCA. These findings suggest the need to further evaluate relationships between cognitively defined late onset Alzheimer’s disease subgroups and these rare Alzheimer’s subtypes. For example, it is unknown whether rates of dyslexia are higher among people with isolated substantial relative language impairment than people with other subtypes of late-onset Alzheimer’s disease. Furthermore, it will be fascinating to see whether people with logopenic variant PPA may have genetic patterns similar to those of people with isolated substantial relative language impairment, and whether people with PCA may have genetic patterns similar to those of people with isolated substantial visuospatial impairment. It will be of great interest in the coming years to elucidate relationships across these conditions. Such investigations may reveal important insights into shared and distinct mechanisms underlying susceptibility to these conditions.
Our results should be considered mindful of limitations of our study. Data evaluated here are from studies with well-educated people of European ancestry. It will be important to replicate this approach among people with diverse genetic backgrounds. While we combined data from five large studies, the resulting subgroups were underpowered to reach genome-wide significance, and one subgroup (isolated substantial relative executive functioning impairment) was too small to analyze at all. It will be important to incorporate additional data sets to see whether novel suggestive loci reach genome-wide significance, and to identify additional loci. We used a large threshold of 0.80 SD to characterize “substantial” relative impairments, which may be too conservative. Our categorization approach relies exclusively on cognitive data. We could imagine a more optimal approach that also incorporates imaging and/or fluid biomarkers.
In conclusion, genome-wide genetic data enabled us to determine that a cognitively defined categorization scheme produced biologically coherent subgroups of people with Alzheimer’s disease. This is an important result on the road towards personalized medicine.
Change history
08 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41380-019-0348-x
References
Lam B, Masellis M, Freedman M, Stuss DT, Black SE. Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res Ther. 2013;5:1.
Cholerton B, Larson EB, Quinn JF, Zabetian CP, Mata IF, Keene CD, et al. Precision medicine: clarity for the complexity of dementia. Am J Pathol. 2016;186:500–6.
Girard SL, Rouleau GA. Genome-wide association study in FTD: divide to conquer. Lancet Neurol. 2014;13:643–4.
Crane PK, Trittschuh E, Mukherjee S, Saykin AJ, Sanders RE, Larson EB, et al. Incidence of cognitively defined late-onset Alzheimer’s dementia subgroups from a prospective cohort study. Alzheimer’s Dement. 2017;13:1307–16.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44.
Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–8.
Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43:436–41.
Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4.
Muthen LK, Muthen BO. Mplus user’s guide. 7th ed. LA: Muthen & Muthen; 1998–2012.
Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–73.
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7.
Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1.
Schott JM, Crutch SJ, Carrasquillo MM, Uphill J, Shakespeare TJ, Ryan NS, et al. Genetic risk factors for the posterior cortical atrophy variant of Alzheimer’s disease. Alzheimers Dement. 2016;12:862–71.
El Haj M, Antoine P, Amouyel P, Lambert JC, Pasquier F, Kapogiannis D. Apolipoprotein E (APOE) epsilon4 and episodic memory decline in Alzheimer’s disease: a review. Ageing Res Rev. 2016;27:15–22.
DeMichele-Sweet MAA, Weamer EA, Klei L, Vrana DT, Hollingshead DJ, Seltman HJ, et al. Genetic risk for schizophrenia and psychosis in Alzheimer disease. Mol Psychiatry. 2017;23:963–72.
Sherva R, Tripodis Y, Bennett DA, Chibnik LB, Crane PK, de Jager PL, et al. Genome-wide association study of the rate of cognitive decline in Alzheimer’s disease. Alzheimers Dement. 2014;10:45–52.
Scheltens NM, Galindo-Garre F, Pijnenburg YA, van der Vlies AE, Smits LL, Koene T, et al. The identification of cognitive subtypes in Alzheimer’s disease dementia using latent class analysis. J Neurol Neurosurg Psychiatry. 2016;87:235–43.
Scheltens NME, Tijms BM, Koene T, Barkhof F, Teunissen CE, Wolfsgruber S, et al. Cognitive subtypes of probable Alzheimer’s disease robustly identified in four cohorts. Alzheimers Dement. 2017;13:1226–36.
Noh Y, Jeon S, Lee JM, Seo SW, Kim GH, Cho H, et al. Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology. 2014;83:1936–44.
Dong A, Toledo JB, Honnorat N, Doshi J, Varol E, Sotiras A, et al. Heterogeneity of neuroanatomical patterns in prodromal Alzheimer’s disease: links to cognition, progression and biomarkers. Brain. 2017;140:735–47.
Ossenkoppele R, Cohn-Sheehy BI, La Joie R, Vogel JW, Moller C, Lehmann M, et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp. 2015;36:4421–37.
Dickerson BC, Wolk DA. Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011;82:45–51.
Mez J, Mukherjee S, Thornton T, Fardo DW, Trittschuh E, Sutti S, et al. The executive prominent/memory prominent spectrum in Alzheimer’s disease is highly heritable. Neurobiol Aging. 2016;41:115–21.
Mukherjee S, Trittschuh E, Gibbons LE, Mackin RS, Saykin A, Crane PK, et al. Dysexecutive and amnesic AD subtypes defined by single indicator and modern psychometric approaches: relationships with SNPs in ADNI. Brain Imaging Behav. 2012;6:649–60.
Mez J, Cosentino S, Brickman AM, Huey ED, Manly JJ, Mayeux R. Dysexecutive versus amnestic Alzheimer disease subgroups: analysis of demographic, genetic, and vascular factors. Alzheimer Dis Assoc Disord. 2013;27:218–25.
Mez J, Cosentino S, Brickman AM, Huey ED, Manly JJ, Mayeux R. Faster cognitive and functional decline in Dysexecutive versus amnestic Alzheimer’s subgroups: a longitudinal analysis of the National Alzheimer’s Coordinating Center (NACC) database. PLoS One. 2013;8:e65246.
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29.
Miller ZA, Mandelli ML, Rankin KP, Henry ML, Babiak MC, Frazier DT, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013;136:3461–73.
Acknowledgements
Analyses were funded by R01 AG042437 (PKC, PI) and R01 AG029672 (PKC, PI). Data archiving was supported by R01 AG042437-S1 (PKC, PI). JM’s efforts were also supported by K23 AG046377 (JM, PI). The efforts of LEG, TJG, and CDK were also supported by P50 AG005136 (TJG, PI). CDK’s efforts were also supported by the Nancy and Buster Alvord Endowment. ALG’s efforts were supported by K01 AG050699 (ALG, PI). TDB’s efforts were supported by Department of Veterans Affairs Research Funds. AJS’s efforts were supported by U01 AG042904 (Weiner M, PI), P30 AG010133 (AJS, PI), and R01 AG019771 (AJS, PI). ACT data collection was supported by U01 AG006781 (Larson E and Crane P, MPIs). ACT genotyping was supported by U01 HG006375 (EBL and G. Jarvik, MPIs). Cerebellum samples for genotyping for some ACT samples were prepared with support from P50 AG005136 (TJG, PI). The authors thank Aimee Schantz and Allison Beller for administrative support for the ACT cerebellum samples. ADNI data collection and genotyping were supported by U01 AG024904 (Weiner M, PI). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. MAP data collection and genotyping were supported by R01 AG017917 (DAB, PI). Additional genotyping was supported by Kronos, Zinfandel, and U01 AG032984 (Schellenberg G, PI). ROS data collection and genotyping were supported by P30 AG10161 (DAB, PI), R01 AG15819 (DAB, PI), and R01 AG30146 (D. Evans, PI). Additional genotyping was supported by Kronos, Zinfandel, and U01 AG032984 (G. Schellenberg, PI). PITT data collection were funded by P50 AG05133 (OLL, PI), R01 AG030653 (MIK, PI), and R01 AG041718 (MIK, PI).
Alzheimer's Disease Neuroimaging Initiative: ADNI
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mukherjee, S., Mez, J., Trittschuh, E. et al. Genetic data and cognitively defined late-onset Alzheimer’s disease subgroups. Mol Psychiatry 25, 2942–2951 (2020). https://doi.org/10.1038/s41380-018-0298-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0298-8
This article is cited by
-
Diagnosis and Management of Posterior Cortical Atrophy
Current Treatment Options in Neurology (2023)
-
A genome-wide search for pleiotropy in more than 100,000 harmonized longitudinal cognitive domain scores
Molecular Neurodegeneration (2023)
-
Recent update on the heterogeneity of the Alzheimer’s disease spectrum
Journal of Neural Transmission (2022)
-
Pre-statistical harmonization of behavioral instruments across eight surveys and trials
BMC Medical Research Methodology (2021)
-
Short leukocyte telomeres predict 25-year Alzheimer's disease incidence in non-APOE ε4-carriers
Alzheimer's Research & Therapy (2021)