Abstract
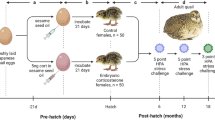
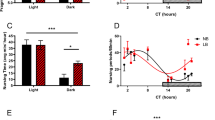
Stressful events in early life might lead to stress resilience or vulnerability, depending on an adjustable stress-response set-point, which can be altered during postnatal sensory development and involves epigenetic regulation of corticotropin-releasing hormone (CRH). During the critical developmental period of thermal-control establishment in 3-day-old chicks, heat stress was found to affect both body temperature and expression of CRH in the hypothalamic paraventricular nucleus. Both increased during heat challenge in vulnerable chicks, whereas they decreased in resilient chicks. Our aim was to elucidate the epigenetic mechanism underlying the regulation of stress resilience or vulnerability. Accordingly, DNA CpG methylation (5mC) and hydroxymethylation (5hmC) at the CRH intron, which we found to serve as a repressor element, displayed low 5mc% alongside high 5hmc% in resilient chicks, and high 5mc% with low 5hmc% in vulnerable ones. RE1-silencing transcription factor (REST), which has a binding site on this intron, bound abundantly during acute heat stress and was nearly absent during moderate stress, restricting repression by the repressor element, and thus activating CRH gene transcription. Furthermore, REST assembled into a protein complex with TET3, which bound directly to the CRH gene. Finally, the adjacent histone recruited the histone acetylation enzyme GCN5 to this complex, which increased H3K27ac during harsh, but not moderate heat conditioning. We conclude that an epigenetic mechanism involving both post-translational histone modification and DNA methylation in a regulatory segment of CRH is involved in determining a resilient or vulnerable response to stress later in life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Callaghan BL, Graham BM, Li S, Richardson R. From resilience to vulnerability: mechanistic insights into the effects of stress on transitions in critical period plasticity. Front Psychiatry. 2013;4:90.
King EC, Pattwell SS, Glatt CE, Lee FS. Sensitive periods in fear learning and memory. Stress. 2014;17:13–21.
Hartley CA, Lee FS. Sensitive periods in affective development: nonlinear maturation of fear learning. Neuropsychopharmacology. 2015;40:50–60.
Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–79.
Claessens SEF, Daskalakis NP, Van Der Veen R, Oitzl MS, De Kloet ER, Champagne DL. Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology (Berl). 2011;214:141–54.
Macrì S, Würbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav. 2006;50:667–80.
Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45.
Franklin TB, Russig H, Weiss IC, Grff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–15.
Yeshurun S, Rogers J, Short AK, Renoir T, Pang TY, Hannan AJ. Elevated paternal glucocorticoid exposure modifies memory retention in female offspring. Psychoneuroendocrinology. 2017;83:9–18.
Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–12.
Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci USA. 2010;107:8507–12.
Korosi A, Shanabrough M, McClelland S, Liu Z-W, Borok E, Gao X-B, et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci. 2010;30:703–13.
Kageyana K, Suda T. Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalam. Endocr J. 2009;56:335–44.
Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–61.
Cramer T, Kisliouk T, Yeshurun S, Meiri N. The balance between stress resilience and vulnerability is regulated by corticotropin-releasing hormone during the critical postnatal period for sensory development. Dev Neurobiol. 2015;75:842–53.
Kisliouk T, Cramer T, Meiri N. Heat stress attenuates new cell generation in the hypothalamus: a role for miR-138. Neuroscience. 2014;277:624–36.
Kisliouk T, Cramer T, Meiri N. Methyl CpG level at distal part of heat-shock protein promoter HSP70 exhibits epigenetic memory for heat stress by modulating recruitment of POU2F1-associated nucleosome-remodeling deacetylase (NuRD) complex. J Neurochem. 2017;141:358–72.
McGowan PO, Suderman M, Sasaki A, Huang TCT, Hallett M, Meaney MJ, et al. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS ONE. 2011;6:e14739.
Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54.
Rudenko A, Tsai LH. Epigenetic modifications in the nervous system and their impact upon cognitive impairments. Neuropharmacology. 2014;80:70–82.
Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351–3.
Gapp K, von Ziegler L, Tweedie-Cullen RY, Mansuy IM. Early life epigenetic programming and transmission of stress-induced traits in mammals: how and when can environmental factors influence traits and their transgenerational inheritance? Bioessays. 2014;36:491–502.
Tabachnik T, Kisliouk T, Marco A, Meiri N, Weller A. Thyroid hormone-dependent epigenetic regulation of melanocortin 4 receptor levels in female offspring of obese rats. Endocrinology. 2017;158:842–51.
Kuenzel WJ, Masson M. A stereotaxic atlas of the brain of the chick (Gallus domesticus). Baltimore: Johns Hopkins University Press; 1988.
Guo JU, Su Y, Zhong C, Ming GL, Song H. Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond. Cell Cycle. 2011;10:2662–8.
Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35:1562–92.
Roman E, Gustafsson L, Berg M, Nylander I. Behavioral profiles and stress-induced corticosteroid secretion in male Wistar rats subjected to short and prolonged periods of maternal separation. Horm Behav. 2006;50:736–47.
Provençal N, Binder EB. The effects of early life stress on the epigenome: from the womb to adulthood and even before. Exp Neurol. 2014;268:10–20.
Molet J, Maras PM, Avishai-Eliner S, Baram TZ. Naturalistic rodent models of chronic early-life stress. Dev Psychobiol. 2014;56:1675–88.
Nishi M, Horii-Hayashi N, Sasagawa T. Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Front Neurosci. 2014;8:166.
Chen Y, Baram TZ. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2016;41:197–206.
Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200.
Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–900.
Tzschentke B. Attainment of thermoregulation as affected by environmental factors. Poult Sci. 2007;86:1025–36.
Tzschentke B, Halle I. Influence of temperature stimulation during the last 4 days of incubation on secondary sex ratio and later performance in male and female broiler chicks. Br Poult Sci. 2009;50:634–40.
Tzschentke B, Basta D. Early development of neuronal hypothalamic thermosensitivity in birds: influence of epigenetic temperature adaptation. Comp Biochem Physiol Part A Mol Integr Physiol. 2002;131:825–32.
Lay DC, Wilson ME. Development of the chicken as a model for prenatal stress. J Anim Sci. 2002;80:1954–61.
Arjona AA, Denbow DM, Weaver WD. Neonatally-induced thermotolerance: physiological responses. Comp Biochem Physiol Part A Physiol. 1990;95:393–9.
Arjona AA, Denbow DM, Weaver WD. Effect of heat stress early in life on mortality of broilers exposed to high environmental temperatures just prior to marketing. Poult Sci. 1988;67:226–31.
Yahav S, Hurwitz S. Induction of thermotolerance in male broiler chickens by temperature conditioning at an early age. Poult Sci. 1996;75:402–6.
Yahav S, McMurtry JP. Thermotolerance acquisition in broiler chickens by temperature conditioning early in life—the effect of timing and ambient temperaturey. Poult Sci. 2001;80:1662–6.
Morimoto A, Nakamori T, Morimoto K, Tan N, Murakami N. The central role of corticotrophin-releasing factor (CRF-41) in psychological stress in rats. J Physiol. 1993;460:221–9.
Füzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun. 2016;7:11937.
Elfwing M, Nätt D, Goerlich-Jansson VC, Persson M, Hjelm J, Jensen P. Early stress causes sex-specific, life-long changes in behaviour, levels of gonadal hormones, and gene expression in chickens. PLoS ONE. 2015;10:e0125808.
McIlwrick S, Rechenberg A, Matthes M, Burgstaller J, Schwarzbauer T, Chen A, et al. Genetic predisposition for high stress reactivity amplifies effects of early-life adversity. Psychoneuroendocrinology. 2016;70:85–97.
McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8.
Bockmühl Y, Patchev AV, Madejska A, Hoffmann A, Sousa JC, Sousa N, et al. Methylation at the CpG island shore region upregulates Nr3c1 promoter activity after early-life stress. Epigenetics. 2015;10:247–57.
Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity (Edinb). 2010;105:4–13.
Bestor TH, Edwards JR, Boulard M. Notes on the role of dynamic DNA methylation in mammalian development. Proc Natl Acad Sci USA. 2015;112:6796–9.
Naumova OY, Lee M, Koposov R, Szyf M, Dozier M, Grigorenko EL. Differential patterns of whole-genome DNA methylation in institutionalized children and children raised by their biological parents. Dev Psychopathol. 2012;24:143–55.
Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND. et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905
Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, Dsouza C, Fouse SD, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–7.
Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;39:457–66.
Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5.
Ito S, Dalessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–33.
Schwanhüusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–42.
Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, et al. Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci USA. 2004;101:10458–63.
Seth KA, Majzoub JA. Repressor Element Silencing Transcription Factor/Neuron-restrictive Silencing Factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin-releasing hormone gene transcription. J Biol Chem. 2001;276:13917–23.
Perera A, Eisen D, Wagner M, Laube SK, Künzel AF, Koch S, et al. TET3 is recruited by REST for context-specific hydroxymethylation and induction of gene expression. Cell Rep. 2015;11:283–94.
Katada S, Imhof A, Sassone-Corsi P. Connecting threads: epigenetics and metabolism. Cell. 2012;148:24–8.
Rinaldi G, Rossi M, Fendt S-M. Metabolic interactions in cancer: cellular metabolism at the interface between the microenvironment, the cancer cell phenotype and the epigenetic landscape. Wiley Interdiscip Rev Syst Biol Med. 2018;10:e1397.
Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, et al. Editing DNA methylation in the mammalian genome. Cell. 2016;167:233–.e17.
Lei Y, Zhang X, Su J, Jeong M, Gundry MC, Huang YH, et al. Targeted DNA methylation in vivo using an engineered dCas9-MQ1 fusion protein. Nat Commun. 2017;8:16026.
Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017;169:559.
Acknowledgements
We are grateful to the Volcani Institute chicken farm staff for their dedicated work. We would like to thank Dr. Shlomo Yeshurun of the Florey Institute of Neuroscience and Mental Health at the University of Melbourne for his valuable comments, input and scientific editing, and Perry Samnon for his assistance in performing some of the experiments. We would like to thank Timna Cramer for the graphic illustration design and production. This work was supported by the Israel Science Foundation (grant no. 1646/15) and The Chief Scientist of the Ministry of Agriculture, Israel (grant no. 3561698). Contribution No. 018/17 from the ARO, the Volcani Center, Rishon LeZiyyon 7528809, Israel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cramer, T., Rosenberg, T., Kisliouk, T. et al. Early-life epigenetic changes along the corticotropin-releasing hormone (CRH) gene influence resilience or vulnerability to heat stress later in life. Mol Psychiatry 24, 1013–1026 (2019). https://doi.org/10.1038/s41380-018-0280-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0280-5
This article is cited by
-
Transgenerational transmission of environmental effects in livestock in the age of global warming
Cell Stress and Chaperones (2023)
-
Effect of acute heat shock on stress gene expression and DNA methylation in zebu (Bos indicus) and crossbred (Bos indicus × Bos taurus) dairy cattle
International Journal of Biometeorology (2022)