Abstract
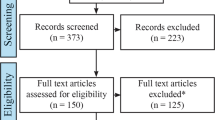
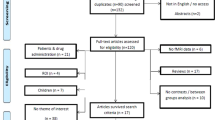
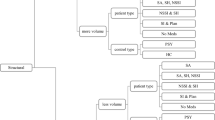
Psychopathy is a disorder of high public concern because it predicts violence and offense recidivism. Recent brain imaging studies suggest abnormal brain activity underlying psychopathic behavior. No reliable pattern of altered neural activity has been disclosed so far. This study sought to identify consistent changes of brain activity in psychopaths and to investigate whether these could explain known psychopathology. First, we used activation likelihood estimation (p < 0.05, corrected) to meta-analyze brain activation changes associated with psychopathy across 28 functional magnetic resonance imaging studies reporting 753 foci from 155 experiments. Second, we characterized the ensuing regions functionally by employing metadata of a large-scale neuroimaging database (p < 0.05, corrected). Psychopathy was consistently associated with decreased brain activity in the right laterobasal amygdala, the dorsomedial prefrontal cortex, and bilaterally in the lateral prefrontal cortex. A robust increase of activity was observed in the fronto-insular cortex on both hemispheres. Data-driven functional characterization revealed associations with semantic language processing (left lateral prefrontal and fronto-insular cortex), action execution and pain processing (right lateral prefrontal and left fronto-insular), social cognition (dorsomedial prefrontal cortex), and emotional as well as cognitive reward processing (right amygdala and fronto-insular cortex). Aberrant brain activity related to psychopathy is located in prefrontal, insular, and limbic regions. Physiological mental functions fulfilled by these brain regions correspond to disturbed behavioral patterns pathognomonic for psychopathy. Hence, aberrant brain activity may not just be an epiphenomenon of psychopathy but directly related to the psychopathology of this disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Koch JLA. Die psychopathischen Minderwertigkeiten. Ravensburg: Maier; 1891.
Schneider K. Die psychopathischen Persönlichkeiten. Wien: Deuticke; 1923.
Cleckley HM. The Mask of Sanity. St. Louis: Mosby; 1941.
Devereux G. Ethnopsychiatry as a frame of reference in clinical research an practice. In: Devereux G. editor. Basic problems of ethnopsychiatry (1980). Chicago, IL: Chicago University Press; 1952.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
World Health Organziation. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organziation; 1992.
Widiger TA. Psychopathy and DSM-IV psychopathology. In: Patrick CJ, editor. Handbook of psychopathy. New York, NY: Guilford Press; 2006.
Hare RD, Neumann CS. Psychopathy as a clinical and empirical construct. Annu Rev Clin Psychol. 2008;4:217–46.
Leistico AM, Salekin RT, DeCoster J, Rogers R. A meta-analysis relating the Hare measures of psychopathy to antisocial conduct. Law Hum Behav. 2008;32:28–45.
Coid J, Yang M. The distribution of psychopathy among a household population: categorical or dimensional? Soc Psychiatry Psychiatr Epidemiol. 2008;43:773–81.
Lösel F. Is effective treatment of psychopathy possible? What we know and what we need to know. In: Raine A, Sanmartín J, editors. Violence and psychopathy. New York, NY: Springer; 2001.
Mokros A, Vohs K, Habermeyer E. Psychopathy and violent reoffending in German-speaking countries: a meta-analysis. Eur J Psychol Assess. 2014;30:117–29.
Koenigs M, Baskin-Sommers A, Zeier J, Newman JP. Investigating the neural correlates of psychopathy: a critical review. Mol Psychiatry. 2011;16:792–9.
Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage. 2002;16:765–80.
Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26.
Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13.
Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–61.
Hare RD. Hare Psychopathy Checklist—revised. 2nd ed. Toronto: Multi-Health Systems; 2003.
Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutiérrez D, Moran ST, Gonzales SM, et al. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage. 2010;51:677–83.
Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 2016;137:70–85.
Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98.
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35.
Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–82.
Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–21.
Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, et al. BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp. 2005;25:185–98.
Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, et al. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage. 2013;81:381–92.
Poeppl TB, Müller VI, Hoffstaedter F, Bzdok D, Laird AR, Fox PT, et al. Imbalance in subregional connectivity of the right temporoparietal junction in major depression. Hum Brain Mapp. 2016;37:2931–42.
Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl). 2005;210:343–52.
Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–41.
Bagley AD, Abramowitz CS, Kosson DS. Vocal affect recognition and psychopathy: converging findings across traditional and cluster analytic approaches to assessing the construct. J Abnorm Psychol. 2009;118:388–98.
Kiehl KA, Hare RD, McDonald JJ, Brink J. Semantic and affective processing in psychopaths: an event-related potential (ERP) study. Psychophysiology. 1999;36:765–74.
Williamson S, Harpur TJ, Hare RD. Abnormal processing of affective words by psychopaths. Psychophysiology. 1991;28:260–73.
Blair KS, Richell RA, Mitchell DG, Leonard A, Morton J, Blair RJ. They know the words, but not the music: affective and semantic priming in individuals with psychopathy. Biol Psychol. 2006;73:114–23.
Krakowski MI, Foxe J, de Sanctis P, Nolan K, Hoptman MJ, Shope C, et al. Aberrant response inhibition and task switching in psychopathic individuals. Psychiatry Res. 2015;229:1017–23.
Kim YY, Jung YS. Reduced frontal activity during response inhibition in individuals with psychopathic traits: an sLORETA study. Biol Psychol. 2014;97:49–59.
Weidacker K, Weidemann CT, Boy F, Johnston SJ. Cathodal tDCS improves task performance in participants high in Coldheartedness. Clin Neurophysiol. 2016;127:3102–9.
Brislin SJ, Buchman-Schmitt JM, Joiner TE, Patrick CJ. “Do unto others”? Distinct psychopathy facets predict reduced perception and tolerance of pain. Personal Disord. 2016;7:240–6.
Decety J, Chen C, Harenski C, Kiehl KA. An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Front Hum Neurosci. 2013;7:489.
Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, et al. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217:783–96.
Contreras-Rodríguez O, Pujol J, Batalla I, Harrison BJ, Soriano-Mas C, Deus J, et al. Functional connectivity bias in the prefrontal cortex of psychopaths. Biol Psychiatry. 2015;78:647–55.
Blair RJ. Reward processing, functional connectivity, psychopathy, and research domain criteria. Biol Psychiatry. 2015;78:592–3.
Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Mol Psychiatry. 2009;14:561–2.
Yang Y, Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biol Psychiatry. 2005;57:1103–8.
Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. J Abnorm Psychol. 2010;119:546–54.
Vieira JB, Ferreira-Santos F, Almeida PR, Barbosa F, Marques-Teixeira J, Marsh AA. Psychopathic traits are associated with cortical and subcortical volume alterations in healthy individuals. Soc Cogn Affect Neurosci. 2015;10:1693–704.
Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142:107–28.
Blair RJR. A cognitive developmental approach to morality: investigating the psychopathy. Cognition. 1995;57:1–29.
Genon S, Reid A, Langner R, Amunts K, Eickhoff SB. How to characterize the function of a brain region. Trends Cogn Sci. 2018;22:350–64.
Acknowledgements
PTF is supported by the National Institute of Mental Health (R01-MH074457). DB is funded by the Deutsche Forschungsgemeinschaft (DFG, BZ2/2-1, BZ2/3-1, and BZ2/4-1; International Research Training Group IRTG2150), Amazon AWS Research Grant, the German National Academic Foundation, and the START-Program of the Faculty of Medicine, RWTH Aachen. SBE is supported by the Deutsche Forschungsgemeinschaft (DFG, EI 816/4-1, EI 816/6-1), the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain,” and the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 7202070 (HBP SGA1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Poeppl, T.B., Donges, M.R., Mokros, A. et al. A view behind the mask of sanity: meta-analysis of aberrant brain activity in psychopaths. Mol Psychiatry 24, 463–470 (2019). https://doi.org/10.1038/s41380-018-0122-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0122-5
This article is cited by
-
Functional Connectivity of the Nucleus Accumbens across Variants of Callous-Unemotional Traits: A Resting-State fMRI Study in Children and Adolescents
Research on Child and Adolescent Psychopathology (2024)
-
How to Advance the Debate on the Criminal Responsibility of Antisocial Offenders
Neuroethics (2024)
-
Asymmetry, cytoarchitectonic morphology and genetics associated with Broca’s area in schizophrenia
Nature Mental Health (2024)
-
Larger left hippocampal presubiculum is associated with lower risk of antisocial behavior in healthy adults with childhood conduct history
Scientific Reports (2023)
-
Aberrant brain activity in pedophilia links to receptor distribution, gene expression, and behavior
Nature Mental Health (2023)