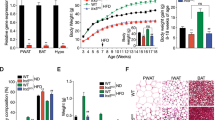
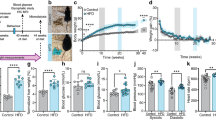
Abstract
Consumption of high fat, high sugar (western) diets is a major contributor to the current high levels of obesity. Here, we used a multidisciplinary approach to gain insight into the molecular mechanisms underlying susceptibility to diet-induced obesity (DIO). Using positron emission tomography (PET), we identified the dorsal striatum as the brain area most altered in DIO-susceptible rats and molecular studies within this region highlighted regulator of G-protein signaling 4 (Rgs4) within laser-capture micro-dissected striatonigral (SN) and striatopallidal (SP) medium spiny neurons (MSNs) as playing a key role. Rgs4 is a GTPase accelerating enzyme implicated in plasticity mechanisms of SP MSNs, which are known to regulate feeding and disturbances of which are associated with obesity. Compared to DIO-resistant rats, DIO-susceptible rats exhibited increased striatal Rgs4 with mRNA expression levels enriched in SP MSNs. siRNA-mediated knockdown of striatal Rgs4 in DIO-susceptible rats decreased food intake to levels comparable to DIO-resistant animals. Finally, we demonstrated that the human Rgs4 gene locus is associated with increased body weight and obesity susceptibility phenotypes, and that overweight humans exhibit increased striatal Rgs4 protein. Our findings highlight a novel role for involvement of Rgs4 in SP MSNs in feeding and DIO-susceptibility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–50.
Wang Y, Baker JL, Hill JO, Dietz WH. Controversies regarding reported trends: has the obesity epidemic leveled off in the United States? Adv Nutr. 2012;3:751–2.
Dietz WH. The response of the US Centers for Disease Control and Prevention to the obesity epidemic. Annu Rev Public Health. 2015;36:575–96.
Schemmel R, Mickelsen O, Tolgay Z. Dietary obesity in rats: influence of diet, weight, age, and sex on body composition. Am J Physiol. 1969;216:373–9.
Fisler JS, Shimizu H, Bray GA. Brain 3-hydroxybutyrate, glutamate, and GABA in a rat model of dietary obesity. Physiol Behav. 1989;45:571–7.
Obst BE, Schemmel RA, Czajka-Narins D, Merkel R. Adipocyte size and number in dietary obesity resistant and susceptible rats. Am J Physiol. 1981;240:E47–53.
Primeaux SD, Barnes MJ, Braymer HD, Bray GA. Sensitivity to the satiating effects of exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes. 2010;34:1427–33.
Allerton TD, Primeaux SD High-fat diet differentially regulates metabolic parameters in obesity-resistant S5B/Pl rats and obesity-prone Osborne-Mendel rats. Can J Physiol Pharmacol 2015;14:1–10.
Pittman DW, Smith KR, Crawley ME, Corbin CH, Hansen DR, Watson KJ, et al. Orosensory detection of fatty acids by obesity-prone and obesity-resistant rats: strain and sex differences. Chem Senses. 2008;33:449–60.
Gilbertson TA, Liu L, Kim I, Burks CA, Hansen DR. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav. 2005;86:681–90.
Schaffhauser AO, Madiehe AM, Braymer HD, Bray GA, York DA. Effects of a high-fat diet and strain on hypothalamic gene expression in rats. Obes Res. 2002;10:1188–96.
Barnes MJ, Holmes G, Primeaux SD, York DA, Bray GA. Increased expression of mu opioid receptors in animals susceptible to diet-induced obesity. Peptides. 2006;27:3292–8.
White CL, Ishii Y, Mendoza T, Upton N, Stasi LP, Bray GA, et al. Effect of a selective OX1R antagonist on food intake and body weight in two strains of rats that differ in susceptibility to dietary-induced obesity. Peptides. 2005;26:2331–8.
Liu X, York DA, Bray GA. Regulation of ghrelin gene expression in stomach and feeding response to a ghrelin analogue in two strains of rats. Peptides. 2004;25:2171–7.
Thanos PK, Kim R, Cho J, Michaelides M, Anderson BJ, Primeaux SD, et al. Obesity-resistant S5B rats showed greater cocaine conditioned place preference than the obesity-prone OM rats. Physiol Behav. 2010;101:713–8.
Thanos PK, Cho J, Kim R, Michaelides M, Primeaux S, Bray G, et al. Bromocriptine increased operant responding for high fat food but decreased chow intake in both obesity-prone and resistant rats. Behav Brain Res. 2010;217:165–70.
White CL, Ishihara Y, York DA, Bray GA. Effect of meta-chlorophenylpiperazine and cholecystokinin on food intake of Osborne-Mendel and S5B/P1 rats. Obesity. 2007;15:624–31.
Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–79.
Tulloch AJ, Murray S, Vaicekonyte R, Avena NM. Neural responses to macronutrients: hedonic and homeostatic mechanisms. Gastroenterology. 2015;148:1205–18.
Primeaux SD, Barnes MJ, Bray GA. Olfactory bulbectomy increases food intake and hypothalamic neuropeptide Y in obesity-prone but not obesity-resistant rats. Behav Brain Res. 2007;180:190–6.
Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61.
Thanos PK, Michaelides M, Gispert JD, Pascau J, Soto-Montenegro ML, Desco M, et al. Differences in response to food stimuli in a rat model of obesity: in-vivo assessment of brain glucose metabolism. Int J Obes. 2008;32:1171–9.
Schiffer WK, Mirrione MM, Dewey SL. Optimizing experimental protocols for quantitative behavioral imaging with 18F-FDG in rodents. J Nucl Med. 2007;48:277–87.
Meibach RC, Glick SD, Ross DA, Cox RD, Maayani S. Intraperitoneal administration and other modifications of the 2-deoxy-d-glucose technique. Brain Res. 1980;195:167–76.
Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JL. A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods. 2003;129:105–13.
Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46.
Samadi P, Gregoire L, Morissette M, Calon F, Hadj Tahar A, Dridi M, et al. mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiol Aging. 2008;29:1040–51.
Thanos PK, Ramalhete RC, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Leptin receptor deficiency is associated with upregulation of cannabinoid 1 receptors in limbic brain regions. Synapse. 2008;62:637–42.
Krumins AM, Barker SA, Huang C, Sunahara RK, Yu K, Wilkie TM, et al. Differentially regulated expression of endogenous RGS4 and RGS7. J Biol Chem. 2004;279:2593–9.
Nirmalan NJ, Harnden P, Selby PJ, Banks RE. Development and validation of a novel protein extraction methodology for quantitation of protein expression in formalin-fixed paraffin-embedded tissues using western blotting. J Pathol. 2009;217:497–506.
Rivero G, Gabilondo AM, Garcia-Sevilla JA, La Harpe R, Morentin B, Javier Meana J. Characterization of regulators of G-protein signaling RGS4 and RGS10 proteins in the postmortem human brain. Neurochem Int. 2010;57:722–9.
Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol Psychiatry. 2012;72:803–10.
Laitinen JT, Jokinen M. Guanosine 5’-(gamma-[35S]thio)triphosphate autoradiography allows selective detection of histamine H3 receptor-dependent G protein activation in rat brain tissue sections. J Neurochem. 1998;71:808–16.
Shimoyama M, De Pons J, Hayman GT, Laulederkind SJ, Liu W, Nigam R, et al. The Rat Genome Database 2015: genomic, phenotypic and environmental variations and disease. Nucleic Acids Res. 2015;43:D743–750.
Aarons AR, Talan A, Schiffer WK. Experimental protocols for behavioral imaging: seeing animal models of drug abuse in a new light. Curr Top Behav Neurosci. 2012;11:93–115.
Stratinaki M, Varidaki A, Mitsi V, Ghose S, Magida J, Dias C, et al. Regulator of G protein signaling is a crucial modulator of antidepressant drug action in depression and neuropathic pain models. Proc Natl Acad Sci USA. 2013;110:8254–9.
Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, et al. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–81.
Blazer LL, Roman DL, Chung A, Larsen MJ, Greedy BM, Husbands SM, et al. Reversible, allosteric small-molecule inhibitors of regulator of G protein signaling proteins. Mol Pharmacol. 2010;78:524–33.
Zhong H, Wade SM, Woolf PJ, Linderman JJ, Traynor JR, Neubig RR. A spatial focusing model for G protein signals. Regulator of G protein signaling (RGS) protien-mediated kinetic scaffolding. J Biol Chem. 2002;278:7278–84.
Lerner TN, Kreitzer AC. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron. 2012;73:347–59.
Lobo MK. Molecular profiling of striatonigral and striatopallidal medium spiny neurons past, present, and future. Int Rev Neurobiol. 2009;89:1–35.
Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–41.
Kenny PJ, Voren G, Johnson PM. Dopamine D2 receptors and striatopallidal transmission in addiction and obesity. Curr Opin Neurobiol. 2013;23:535–8.
Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015;8:1–31.
Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–730.
Burchett SA, Bannon MJ, Granneman JG. RGS mRNA expression in rat striatum: modulation by dopamine receptors and effects of repeated amphetamine administration. J Neurochem. 1999;72:1529–33.
Stanwood GD, Parlaman JP, Levitt P. Genetic or pharmacological inactivation of the dopamine D1 receptor differentially alters the expression of regulator of G-protein signalling (Rgs) transcripts. Eur J Neurosci. 2006;24:806–18.
Schwendt M, Gold SJ, McGinty JF. Acute amphetamine down-regulates RGS4 mRNA and protein expression in rat forebrain: distinct roles of D1 and D2 dopamine receptors. J Neurochem. 2006;96:1606–15.
Taymans JM, Kia HK, Claes R, Cruz C, Leysen J, Langlois X. Dopamine receptor-mediated regulation of RGS2 and RGS4 mRNA differentially depends on ascending dopamine projections and time. Eur J Neurosci. 2004;19:2249–60.
Geurts M, Hermans E, Maloteaux JM. Opposite modulation of regulators of G protein signalling-2 RGS2 and RGS4 expression by dopamine receptors in the rat striatum. Neurosci Lett. 2002;333:146–50.
Taymans JM, Leysen JE, Langlois X. Striatal gene expression of RGS2 and RGS4 is specifically mediated by dopamine D1 and D2 receptors: clues for RGS2 and RGS4 functions. J Neurochem. 2003;84:1118–27.
Schwendt M, Sigmon SA, McGinty JF. RGS4 overexpression in the rat dorsal striatum modulates mGluR5- and amphetamine-mediated behavior and signaling. Psychopharmacology. 2012;221:621–35.
Schwendt M, McGinty JF. Regulator of G-protein signaling 4 interacts with metabotropic glutamate receptor subtype 5 in rat striatum: relevance to amphetamine behavioral sensitization. J Pharmacol Exp Ther. 2007;323:650–7.
Dripps IJ, Wang Q, Neubig RR, Rice KC, Traynor JR, Jutkiewicz EM. The role of regulator of G protein signaling 4 in delta-opioid receptor-mediated behaviors. Psychopharmacology. 2016;234:29–39.
Park SW, Shen X, Tien LT, Roman R, Ma T. Methamphetamine-induced changes in the striatal dopamine pathway in mu-opioid receptor knockout mice. J Biomed Sci. 2011;18:83.
Shen W, Plotkin JL, Francardo V, Ko WK, Xie Z, Li Q, et al. M4 muscarinic receptor signaling ameliorates striatal plasticity deficits in models of L-DOPA-induced dyskinesia. Neuron. 2015;88:762–73.
Ding J, Guzman JN, Tkatch T, Chen S, Goldberg JA, Ebert PJ, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9:832–42.
Krashes MJ, Kravitz AV. Optogenetic and chemogenetic insights into the food addiction hypothesis. Front Behav Neurosci. 2014;8:57.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Acknowledgements
This work was supported by the NIAAA (AA11034, AA07574, AA07611, Y1AA3309), the NIDA (DA006278, DA015446, DA023214, DA030359, ZIA000069) and the NINDS (NS086444, NS093537). MM was supported by DA007135. MLM was supported by GM007280 and DA038954. MM is a cofounder and owns stock in Metis Laboratories.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Michaelides, M., Miller, M.L., Egervari, G. et al. Striatal Rgs4 regulates feeding and susceptibility to diet-induced obesity. Mol Psychiatry 25, 2058–2069 (2020). https://doi.org/10.1038/s41380-018-0120-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0120-7
This article is cited by
-
Dopamine D2 receptor overexpression in the nucleus accumbens core induces robust weight loss during scheduled fasting selectively in female mice
Molecular Psychiatry (2021)
-
Obesity: global epidemiology and pathogenesis
Nature Reviews Endocrinology (2019)