Abstract
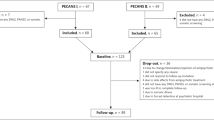
Patients with psychotic disorders are at high risk for type 2 diabetes mellitus, and there is increasing evidence that patients display glucose metabolism abnormalities before significant antipsychotic medication exposure. In the present study, we examined insulin action by quantifying insulin sensitivity in first-episode psychosis (FEP) patients and unaffected siblings, compared to healthy individuals, using a physiological-based model and comprehensive assessment battery. Twenty-two unaffected siblings, 18 FEP patients, and 15 healthy unrelated controls were evaluated using a 2-h oral glucose tolerance test (OGTT), with 7 samples of plasma glucose and serum insulin concentration measurements. Insulin sensitivity was quantified using the oral minimal model method. Lipid, leptin, free fatty acids, and inflammatory marker levels were also measured. Anthropometric, nutrient, and activity assessments were conducted; total body composition and fat distribution were determined using whole-body dual-energy X-ray absorptiometry. Insulin sensitivity significantly differed among groups (F = 6.01 and 0.004), with patients and siblings showing lower insulin sensitivity, compared to controls (P = 0.006 and 0.002, respectively). Body mass index, visceral adipose tissue area (cm2), lipids, leptin, free fatty acids, inflammatory markers, and activity ratings were not significantly different among groups. There was a significant difference in nutrient intake with lower total kilocalories/kilogram body weight in patients, compared to siblings and controls. Overall, the findings suggest that familial abnormal glucose metabolism or a primary insulin signaling pathway abnormality is related to risk for psychosis, independent of disease expression and treatment effects. Future studies should examine underlying biological mechanisms of insulin signaling abnormalities in psychotic disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36:239–45.
Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25:83–8.
Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72:1172–81.
De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77.
Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70:931–9.
Perry BI, McIntosh G, Weich S, Singh S, Rees K. The association between first-episode psychosis and abnormal glycaemic control: systematic review and meta-analysis. Lancet Psychiatry. 2016;3:1049–58.
Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:261–9.
Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71:1350–63.
Rajkumar AP, Horsdal HT, Wimberley T, Cohen D, Mors O, Borglum AD, et al. Endogenous and antipsychotic-related risks for diabetes mellitus in young people with schizophrenia: a Danish population-based cohort study. Am J Psychiatry. 2017;174:686–94.
Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2:452–64.
Henderson DC. Weight gain with atypical antipsychotics: evidence and insights. J Clin Psychiatry. 2007;68:18–26.
Henderson DC, Cagliero E, Copeland PM, Borba CP, Evins E, Hayden D, et al. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005;62:19–28.
Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2012: CD008016.
Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. 2013;47:197–207.
Vancampfort D, Probst M, Knapen J, Carraro A, De Hert M. Associations between sedentary behaviour and metabolic parameters in patients with schizophrenia. Psychiatry Res. 2012;200:73–8.
Crawford MJ, Jayakumar S, Lemmey SJ, Zalewska K, Patel MX, Cooper SJ, et al. Assessment and treatment of physical health problems among people with schizophrenia: national cross-sectional study. Br J Psychiatry. 2014;205:473–7.
Mothi SS, Tandon N, Padmanabhan J, Mathew IT, Clementz B, Tamminga C, et al. Increased cardiometabolic dysfunction in first-degree relatives of patients with psychotic disorders. Schizophr Res. 2015;165:103–7.
van Welie H, Derks EM, Verweij KH, de Valk HW, Kahn RS, Cahn W. The prevalence of diabetes mellitus is increased in relatives of patients with a non-affective psychotic disorder. Schizophr Res. 2013;143:354–7.
Liu Y, Li Z, Zhang M, Deng Y, Yi Z, Shi T. Exploring the pathogenetic association between schizophrenia and type 2 diabetes mellitus diseases based on pathway analysis. BMC Med Genomics. 2013;6:S17.
Lin PI, Shuldiner AR. Rethinking the genetic basis for comorbidity of schizophrenia and type 2 diabetes. Schizophr Res. 2010;123:234–43.
Hansen T, Ingason A, Djurovic S, Melle I, Fenger M, Gustafsson O, et al. At-risk variant in TCF7L2 for type II diabetes increases risk of schizophrenia. Biol Psychiatry. 2011;70:59–63.
Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–46.
Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19:527–34.
Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007;293:E1–15.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Morris AD, Ueda S, Petrie JR, Connell JM, Elliott HL, Donnelly R. The euglycaemic hyperinsulinaemic clamp: an evaluation of current methodology. Clin Exp Pharmacol Physiol. 1997;24:513–8.
Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014;63:1203–13.
Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96.
Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med. 2007;24:481–5.
Enez Darcin A, Yalcin Cavus S, Dilbaz N, Kaya H, Dogan E. Metabolic syndrome in drug-naive and drug-free patients with schizophrenia and in their siblings. Schizophr Res. 2015;166:201–6.
Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92.
Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45.
Shinn AK, Bolton KW, Karmacharya R, Lewandowski KE, Yuksel C, Baker JT, et al. McLean OnTrack: a transdiagnostic program for early intervention in first-episode psychosis. Early Interv Psychiatry. 2017;11:83–90.
First MB, Sptizer RL, Gibbon M, Williams JBW, editors. Structured clinical interview or DSM-IV Axis I Disorders. New York: New York State Pscyhiatric Institute, Biometrics Research; 1995.
Marder SR, Fenton W. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9.
Gross GM, Silvia PJ, Barrantes-Vidal N, Kwapil TR. The dimensional structure of short forms of the Wisconsin Schizotypy Scales. Schizophr Res. 2015;166:80–5.
Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull. 1973;9:13–28.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Beck AT, Steer RA, Brown GK, editors. Manual for the beck depression inventory second edition (BDI-II). The Psychological Corporation, San Antonio, TX. 1996.
Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA, editors. Manual for the state-trait anxiety inventory. Palo Alto: Consulting Psychologists Press; 1983.
Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull. 1998;24:1–10.
Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7.
Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–93.
Basu R, Di Camillo B, Toffolo G, Basu A, Shah P, Vella A, et al. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E55–69.
Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287:E637–43.
Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, et al. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 2005;54:3265–73.
1983 metropolitan height and weight tables. Stat Bull Metrop Life Found 1983; 64 : 3-9.
Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–71.
Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9.
Hallal PC, Victora CG. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med Sci Sports Exerc. 2004;36:556.
Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205.
Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–50.
VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology. 2014;25:749–61.
Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, et al. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55:2001–14.
Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–200.
Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–72.
Du F, Cooper AJ, Thida T, Sehovic S, Lukas SE, Cohen BM, et al. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiatry. 2014;71:19–27.
Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int Rev Neurobiol. 2004;59:273–96.
Yuksel C, Du F, Ravichandran C, Goldbach JR, Thida T, Lin P, et al. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol Psychiatry. 2015;20:1079–84.
Chouinard VA, Kim SY, Valeri L, Yuksel C, Ryan KP, Chouinard G, et al. Brain bioenergetics and redox state measured by 31P magnetic resonance spectroscopy in unaffected siblings of patients with psychotic disorders. Schizophr Res. 2017;187:11–6. https://doi.org/10.1016/j.schres.2017.02.024
Kim SY, Cohen BM, Chen X, Lukas SE, Shinn AK, Yuksel AC, et al. Redox dysregulation in schizophrenia revealed by in vivo NAD + /NADH measurement. Schizophr Bull. 2017;43:197–204.
Bond DJ, Ha TH, Lang DJ, Su W, Torres IJ, Honer WG, et al. Body mass index-related regional gray and white matter volume reductions in first-episode mania patients. Biol Psychiatry. 2014;76:138–45.
Chouinard VA, Pingali SM, Chouinard G, Henderson DC, Mallya SG, Cypess AM, et al. Factors associated with overweight and obesity in schizophrenia, schizoaffective and bipolar disorders. Psychiatry Res. 2016;237:304–10.
Hajek T, Calkin C, Blagdon R, Slaney C, Alda M. Type 2 diabetes mellitus: a potentially modifiable risk factor for neurochemical brain changes in bipolar disorders. Biol Psychiatry. 2015;77:295–303.
Calkin CV, Ruzickova M, Uher R, Hajek T, Slaney CM, Garnham JS, et al. Insulin resistance and outcome in bipolar disorder. Br J Psychiatry. 2015;206:52–7.
Muller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:372.
Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–9.
Kohlgruber AC, LaMarche NM, Lynch L. Adipose tissue at the nexus of systemic and cellular immunometabolism. Semin Immunol. 2016;28:431–40.
Acknowledgements
The authors would like to gratefully acknowledge patients and their families who participated in this study. This research was supported by NIMH to Dost Öngür (R01MH094594 and K24MH104449) and Virginie-Anne Chouinard (5T32MH016259), and the Dupont-Warren and Livingston Fellowships and Maria Lorenz Pope Fellowship to Virginie-Anne Chouinard. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102), financial contributions from Harvard University and its affiliated academic healthcare centers and the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and DK-075116. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DH has received research grants from Otsuka Pharmaceuticals and Reckitt Benckiser. AMC is a recipient of sponsored research grants from Chugai Pharmaceutical Co., Ltd, and Molecular Metabolism, LLC, both through Joslin Diabetes Center; an honorarium for lecturing about brown fat to Pfizer, Inc.; and he has received payment for lecturing about clinical diabetes on behalf of Joslin Diabetes Center to employees of Sanofi, Genentech, Eli Lilly, Janssen, and Regeneron. DÖ was on Scientific Advisory Board for Neurocrine Inc. in 2017. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chouinard, VA., Henderson, D.C., Dalla Man, C. et al. Impaired insulin signaling in unaffected siblings and patients with first-episode psychosis. Mol Psychiatry 24, 1513–1522 (2019). https://doi.org/10.1038/s41380-018-0045-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0045-1
This article is cited by
-
Glucose dysregulation in antipsychotic-naive first-episode psychosis: in silico exploration of gene expression signatures
Translational Psychiatry (2024)
-
Antipsychotics and Medical Comorbidity: A Retrospective Study in an Urban Outpatient Psychiatry Clinic
Community Mental Health Journal (2023)
-
Magnetic Resonance Spectroscopy Studies of Brain Energy Metabolism in Schizophrenia: Progression from Prodrome to Chronic Psychosis
Current Psychiatry Reports (2023)
-
A cross-sectional MR study of body fat volumes and distribution in chronic schizophrenia
Schizophrenia (2022)
-
Metabolic disturbances, hemoglobin A1c, and social cognition impairment in Schizophrenia spectrum disorders
Translational Psychiatry (2022)