Abstract
Substantial variability exists in what pathologists consider as pT4a in colorectal cancer when tumor cells are within 1 mm of the free peritoneal surface. This study aimed to determine if the measured sub-millimeter distance between tumor cells and the free peritoneal surface would offer an objective means of stratifying patients according to the risk of developing peritoneal metastases. Histological slides of patients included in the COLOPEC trial, with resectable primary c/pT4N0-2M0 colon cancer, were centrally reassessed. Specific tumor morphological variables were collected, including distance from tumor to free peritoneal surface, measured in micrometers (µm). The primary outcome, 3-year peritoneal metastasis rate, was compared between four groups of patients stratified for relation of tumor cells to the peritoneum: 1) Full peritoneal penetration with tumor cells on the peritoneal surface, 2) 0–99 µm distance to the peritoneum, 3) 100–999 µm to the peritoneum, and 4) ≥1000 µm to the peritoneum, by using Kaplan-Meier analysis. In total, 189 cases were included in the present analysis. Cases with full peritoneal penetration (n = 89), 0–99 µm distance to the peritoneal surface (n = 34), 100–999 µm distance (n = 33), and ≥1000 µm distance (n = 33), showed significantly different 3-year peritoneal metastases rates of 25% vs 29% vs 6% vs 12%, respectively (Log Rank, p = 0.044). N-category did not influence the risk of peritoneal metastases in patients with a tumor distance beyond 100 µm, while only the N2 category seemed to result in an additive risk in patients with a distance of 0–99 µm. The findings of this study suggest that the measured shortest distance between tumor cells and the free peritoneal surface is useful as an objective means of stratifying patients according to the risk of developing peritoneal metastases. This simple measurement is practical and may help in providing a precise definition of pT4a. Trial registration: NCT02231086 (Clinicaltrials.gov).
Similar content being viewed by others
Introduction
The T category of the TNM system1, representing local invasion, has therapeutic consequences for colon cancer patients regarding the type of resection and whether to offer patients adjuvant therapy, and also has prognostic implications. The most advanced T category (T4) is also considered to be one of the most important risk factors for developing peritoneal metastases, which in turn carry a particularly poor prognosis2,3,4,5. Two main types of locally advanced growth are defined and categorized as pT4a (peritoneal penetration) and pT4b (adjacent organ/structure invasion)1. There is accumulating data that the pT4a subcategory is diagnostically less straightforward than often assumed6,7. This problem has been highlighted in recent studies investigating interobserver variability in diagnosing pT4a8,9,10. It is also reflected in considerable divergence in published rates of pT4 ranging from 12% to 55% for stage I–III colorectal cancer combined11,12,13.
The underlying problem of the pT4a category retains how the cutoff between pT3 and pT4a should be defined. Is full peritoneal penetration with cancer cells being present on the surface required, or should cases with tumor cells variably close to or at the peritoneal surface also be regarded as pT4a? This question is analogous to other problems in colorectal pathology, such as which distance should be used when defining a positive circumferential resection margin (CRM) in rectal carcinoma (0, ≤1, or ≤2 mm). To answer such questions, it is necessary to assess various types of clinical outcome measures that are relevant for a certain parameter.
With regard to the pT4a parameter, the question arises if it should predict overall or disease-free survival, or more specifically the risk of peritoneal recurrence. Furthermore, it is of importance that parameters that have clinical implications should be reliable and reproducible. Prior research has shown that there is wide survival variation within the spectrum of pT3 through pT4a colon cancer and that gradually increasing invasion depth beyond the muscularis propria is associated with worsening of survival14,15. As tumors grow larger and deeper, likely the risk of distant metastases via lymphovascular spread increases and also the risk of peritoneal dissemination when tumor cells breach the peritoneal covering. Studies focusing on the differentiation between pT3 versus pT4a have shown that, when cancer cells are in close proximity to the peritoneal surface, survival becomes gradually more akin to cases showing full peritoneal penetration7,16,17,18. However, different criteria for including these “sub-pT4a” cases within the pT4a category have been put forward. These include the local peritoneal involvement (LPI) classification system of Shepherd18, peritoneal elastic lamina invasion19, and the 1 mm cutoff value (provided that certain fibroinflammatory changes are present)7,16. Based on mainly the Shepherd classification and variants thereof, attempts have been made to further define the pT4a category in national pathology guidelines and review papers8. However, variation in guidelines and subjectivity of the proposed criteria have probably contributed to the current diagnostic ambiguity for pathologists in differentiating between pT3 and pT4a. It is therefore warranted that more objective criteria are provided to standardize this differentiation.
Measurement of the distance between tumor cells and the peritoneal surface can be objectively performed by any pathologist, digitally as well as manually under the microscope. Previously it has been suggested that the 1 mm cutoff value can be used to differentiate between pT3 and pT4a categories, provided that certain fibroinflammatory changes are also present. However, we assumed that this cut-off value might be lower if the pT4a category is specifically used to predict the risk of developing peritoneal metastases. This study aims to clarify the prognostic importance of four different categories regarding the relationship of tumor cells to the peritoneal surface, i.e., “full penetration” with tumor cells on the peritoneal surface, 0–99 micrometers (µm) distance, 100–999 µm distance, and ≥1000 µm distance, with regard to peritoneal metastases and overall survival, in patients with locally advanced colon cancer.
Materials and methods
Study design and patient cohort
This is a side study of the COLOPEC multicenter randomized controlled trial (NCT02231086). The study design and results of the primary endpoint of this trial have been published previously20. Patients were included in the COLOPEC trial between April 1st, 2015 and February 20th, 2017, if they had primary, clinically (preoperative) or pathological proven (postoperative) T4N0-2M0 colon cancer, or colon cancer that had caused a bowel perforation. Other criteria were age between 18 and 75 years, an adequate clinical condition for HIPEC, and intention to start adjuvant systemic chemotherapy. Eligible patients were randomized to the experimental arm comprising adjuvant HIPEC followed by standard adjuvant systemic chemotherapy or the control arm consisting of adjuvant systemic chemotherapy alone.
All histological slides of the primary colon carcinomas of patients included in the COLOPEC trial were requested from the tissue archives of laboratories affiliated with the treating hospital. Patients’ demographics, and baseline characteristics were obtained from the COLOPEC dataset. Additional histopathological specifications were obtained from original pathology reports. All histological slides were reassessed by one pathologist (PS), specialized in colorectal cancer, according to a uniform protocol without any knowledge of the patients’ individual outcomes. Patients that appeared to already have peritoneal metastases during revision were excluded from further analyses, as well as patients who were lost to follow-up.
Review of histopathological characteristics
The slides were scanned (Leica Aperio AT2, ×20 or ×40) and uploaded in our virtual electronic pathology database. During central revision, the following morphological variables were assessed based on standardized criteria: the shortest distance of tumor cells to the free peritoneal surface measured in micrometers (µm), morphological characteristics of the peritoneum/ subperitoneal tissue, resection margin status, pT4b category with type of invaded organ/structure, tumor type and differentiation, tumor border configuration, tumor budding (Bd), tumor-stroma ratio (TSR), venous invasion, lymphatic invasion, perineural invasion, lymph node (LN) status, tumor deposits (TD), and peritoneal metastases.
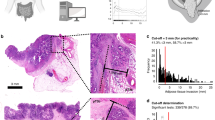
The shortest distance from the neoplastic epithelial tumor cells (referred to in this paper as tumor cells) to the free peritoneal surface in µm, irrespective of the type of intervening stroma/tissue or peritoneal changes, was measured digitally. Relation of tumor cells to the free peritoneal surface was registered irrespective of pT4b status. Cases with tumor cells in direct contact with the peritoneal cavity through a perforation (either via perforated peritumoral abscess or direct tumor perforation by necrosis) were classified as full penetration. The relation between tumor cells and the peritoneal surface was categorized into four groups: (1) Full peritoneal penetration with tumor cells clearly growing onto the peritoneal surface. (2) Shortest distance of 0–99 µm. (3) Shortest distance of 100–999 µm. (4) Shortest distance of ≥1000 µm. Examples are shown in Fig. 1. The choice of the 99/100 µm cut-off value is based on our previous experience in applying the Shepherd's LPI classification in which we demonstrated that a subgroup of LPI3 tumors, i.e., those approaching the LPI4 category, had most similarity to LPI4 with regard to prognosis17. For Shepherd's LPI classification see Suppl. Table 1. The 999/1000 µm represents the cutoff value previously proposed in the literature7,16. Cases where the tumor cells were clearly remote to the free surface, but any relation to the peritoneum was absent in the slides, were classified in category 4 (shortest distance to free peritoneal surface ≥1000 µm). Since 0.1 mm is a more common cutoff point for measurements in pathology, we additionally performed analyses with the four categories combined into two categories <99 µm and ≥100 µm (i.e., <0.1 mm versus ≥0.1 mm).
A, B (×20) are from cases with full peritoneal penetration where the tumor cells were growing onto the peritoneal surface within peritoneal clefts (in case B with inked surface). C (x20) demonstrates a case where the tumor cells reached directly to the free peritoneal surface (distance of 0 µm, arrow). D (×20), E (×20), and F (×4) show cases where the shortest distance of tumor cells to the free peritoneal surface was 60 µm, 167 µm, and 703 µm, respectively (black lines show the location of the measurements).
Morphological characteristics of the peritoneum/subperitoneal tissue were registered as absent or present according to Panarelli et al.: fibroinflammatory tissue reaction, vascular proliferation at the peritoneum, peritumoral abscesses communicating with the peritoneum, reactive mesothelial cells, peritoneal hemorrhage, or peritoneal fibrin deposition16. Resection margin status was defined as positive (R1) when tumor cells extended directly into the resection margin in any of the following scenarios: tumor cells growing into non-peritonealized mesocolic resection margin, tumor cells growing into non-peritonealized resection margin of en-bloc resected organ(s)/structure(s), tumor cells growing into specimen surface at the site of adhesiolysis (as determined by correlation with surgical report), represented by very irregular surface, typically with crushed tissue (mechanical changes), and/or fresh hemorrhage and lacking inflammatory response, and tumor cells extending into the non-peritonealized outer surface of the specimen at the site of a laceration, tear or tissue defect that have occurred either during (e.g., through traction/manipulation) or after the operation (timing usually unknown).
Tumor type and differentiation were categorized as well/moderately differentiated adenocarcinoma, poorly differentiated adenocarcinoma, mucinous carcinoma, and signet ring cell carcinoma according to the WHO criteria. Grading of differentiation was restricted to the extent of gland formation within conventional adenocarcinomas. Tumor border configuration was classified as either pushing or infiltrating as described by Wöhlke et al.21 Tumor budding was defined as low, intermediate or high as described by Lugli et al.22. Signet ring cell carcinomas were classified as high budding (Bd3). TSR was classified as ≤50% stroma and >50% stroma as described by Pelt et al.23. Venous, lymphatic and perineural invasion were assessed using conventional methodology, mainly on HE-slides, and where available using immunohistochemical or elastic stainings. Either intra- and/or extramural venous invasion was registered as present and defaulted to absent in case of doubt. Either intra- and/or extramural lymphatic invasion and intra- and/or extramural perineural invasion were registered as present.
The number of positive LN and TD were registered separately. The differentiation between LN metastases and TD was based on whether there was recognizable lymph node background present. LN status was categorized into pN, pN1 (1–3 positive LN), and pN2 (4 or more positive LN). Mesocolic metastases were defined as TD if there was no recognizable LN background and when larger than 3 mm, according to the TNM-5 definition of TD24, which was still adhered to in the Netherlands due to criticism of the TNM-6 and TNM-7 when the COLOPEC trial started25. Tumor deposits were categorized as absent or present.
Lesions were classified as peritoneal metastases mainly based on location. Location well separate from the primary tumor, based on either pathology request form (separate container) or gross description (typically separate nodules from the greater omentum) was considered indicative for peritoneal metastases. Lesions with epicenter inside the mesocolic fatty tissue were regarded as regular (intramesocolic) TD. Also, lesions were regarded as TD when containing vascular invasion, perivascular growth, or perineural invasion. When in doubt whether a lesion represented a peritoneal metastasis or not, the parameter was defaulted to absent.
Follow-up and endpoints
Follow-up within the COLOPEC trial consisted of imaging of the liver (ultrasound/CT) at 6 and 12 months and standardized CT abdomen at 18 months, combined with blood CEA testing at 3–6 months intervals, during the first 18 months. A diagnostic laparoscopy was performed for peritoneal staging at 18 months if the following criteria were met: the patient was willing to undergo this study procedure, there were no signs of recurrence and patients should be eligible for curative intent treatment. After 18 months and still no signs of recurrence, patients were followed by yearly liver ultrasound/CT abdomen and CEA testing at 6–12 months intervals until 5 years after primary tumor resection.
Our main outcome parameter of the present study was the 3-year metachronous peritoneal metastasis rate. The primary endpoint of the COLOPEC trial was peritoneal metastasis-free survival at 18 months and was assessed centrally, which included imaging of the abdomen with CT, combined with carcinoembryonic antigen testing at 3–6 months intervals. A diagnostic laparoscopy was done at 18 months in all patients without prior incurable, recurrent disease. Metachronous peritoneal metastases from 18 months up till 3-year after the primary resection were detected by routine follow-up according to the Dutch Guidelines, which included yearly liver ultrasound or CT imaging of the abdomen, in combination with serum carcinoembryonic antigen measurement, starting at 24 months after the initial tumor resection. Ovarian and omental metastases were considered as peritoneal metastases as well. Our secondary outcome parameter was 3-year overall survival.
Statistical analysis
Descriptive statistics were used to present patient-, procedure-, and basic tumor characteristics, and tumor morphology characteristics. Patients were stratified by the four groups of shortest distance from the tumor to the peritoneal surface. Categorical data were presented as numbers with percentages and compared using the chi-square test. Continuous data were presented as means with standard deviations and statistically significant differences were assessed using parametric tests for data with a normal distribution. Metachronous peritoneal metastasis rate was determined using Kaplan-Meier analysis, and differences between patient groups were assessed using Log Rank test. Sensitivity analysis of the association between tumor distance from the peritoneal surface and peritoneal metastasis rate was performed for subgroups with R0 resection, N stage, and tumor type other than signet ring cell carcinoma. Significance level was set at a p value of 0.05. All analyses were performed using Statistical Package for Social Sciences version 26.0 (IBM Corp. Armonk, New York, United States of America).
Results
Patient cohort
Out of 202 patients in the COLOPEC trial, 189 were included in the present study (Suppl. Fig. 1). Reasons for exclusions were as follows: non-resectable tumor (n = 1), liver metastases found during primary resection (n = 1), histological slides unavailable (n = 1), and patients who appeared to have synchronous peritoneal metastases found during revision (n = 10). In 89 patients, the tumor cells clearly penetrated the peritoneal surface, 34 had a distance of 0–99 µm to the free peritoneal surface, 33 had a distance of 100–999 µm, and 33 had a distance of ≥1000 µm. Patient-, procedure-, and basic tumor characteristics are presented in Table 1. There was no difference in the percentage of patients that received adjuvant chemotherapy or adjuvant HIPEC among the four groups.
Histopathological characteristics
Table 2 presents histopathological characteristics of the primary tumor, for all patients in total and stratified by the relation of nearest tumor cells to the peritoneal surface. Full peritoneal penetration correlated with more aggressive pathology profile reflected by higher percentages of multiple features such as signet ring cell tumor type, infiltrating tumor border configuration, high tumor budding (Bd3) and lymph node status. Peritoneal changes as defined by Panarelli, et al.16 were present in most cases (87%) but were least common in the category of tumors with a distance of tumor cells to the surface of ≥1000 µm (45%). The presence of pT4b occurred more often in cases where the distance of tumor cells to the free peritoneal surface was 100–999 and ≥1000 µm.
Univariable analysis for metachronous peritoneal metastases
Median follow-up was 36 months (interquartile range 24–48). Crude rates of metachronous peritoneal metastases that were diagnosed during follow-up for the different tumor and histopathological characteristics are presented in Table 3. The measured relationship of tumor cells to the free peritoneal surface was significantly associated with the occurrence of peritoneal metastases (p = 0.038). Peritoneal metastases occurred in 22 of 89 and 10 of 34 patients where tumor cells either penetrated onto the free peritoneal surface or were found very close to the free surface with a of 0–99 µm distance, respectively. When the shortest distance to the peritoneal surface was 100–999 µm or ≥1000 µm, 2 of 33 and 4 of 33 patients developed peritoneal metastases, respectively. The two patients belonging to the 100–999 µm category who developed peritoneal metastases had measured distances of 940 µm and 524 µm, and the former was a R1 resection. Of four patients belonging to the ≥1000 µm category, two patients had an R1 resection.
Among the other histopathological characteristics, higher crude rates of peritoneal metastases were found for infiltrating tumor border configuration 32/134 (23.9%), lymphatic invasion 23/78 (29.5%), perineural invasion 28/103 (27.2%), 20/72 pN2 category (27.8%), signet ring cell carcinoma 5/11 (45.5%). The overall crude peritoneal metastasis rate of R0 (32/164) and R1 (6/25) resections was not significantly different (19.5% vs 24.0%, p = 0.602).
Since lymph node status has previously been described as an important risk factor for developing peritoneal metastases, it was combined with the distance parameter (Suppl. Table 2). This analysis indicated that N1 status was not influencing peritoneal metastasis rate and that the pN2 category seemed to increase the risk of peritoneal metastases when there was either full or close to full penetration (0–99 µm distance) present (38/55, 33%), but not when the tumor cells were further away from the peritoneal surface (2/17, 12%).
3-year risk of peritoneal metastases and overall survival
The 3-year peritoneal metastases rates in patients with full peritoneal penetration were 25%, and this was 29% for tumors with a distance of 0–99 µm, 6% for a distance of 100–999 µm and 12% for a distance ≥1000 µm, which reached statistical significance overall (Log Rank, p = 0.044, Fig. 2A). When excluding all patients with R1 resection, this difference remained (Log Rank, p = 0.046, Fig. 2B). After excluding signet ring cell carcinomas, the curves remained separated, although not significantly different (Log Rank, p = 0.075, Fig. 2C). After exclusion of pT4b tumors, the curves remained separated, although not significantly different (Log Rank, p = 0.205, Fig. 2D). There was no significant difference in 3-year overall survival between the four groups of tumor distances (Fig. 3).
A Kaplan Meier analysis for 3-year risk of peritoneal metastases stratified by the measured relationship of tumor cells to the peritoneum, n = 189. μm micrometers, PM peritoneal metastases.  B Kaplan Meier subgroup analysis for 3-year risk of peritoneal metastases of all patients with a R0 resection, stratified by tumor distance to the peritoneum, n = 164. μm micrometers, PM peritoneal metastases.
B Kaplan Meier subgroup analysis for 3-year risk of peritoneal metastases of all patients with a R0 resection, stratified by tumor distance to the peritoneum, n = 164. μm micrometers, PM peritoneal metastases.  C Kaplan Meier subgroup analysis for 3-year risk of peritoneal metastases of all patients without signet ring cell histology, stratified by tumor distance to the peritoneum, n = 178. μm micrometers, PM peritoneal metastases.
C Kaplan Meier subgroup analysis for 3-year risk of peritoneal metastases of all patients without signet ring cell histology, stratified by tumor distance to the peritoneum, n = 178. μm micrometers, PM peritoneal metastases.  D Kaplan Meier analysis for 3-year risk of peritoneal metastases of all patients without pT4b tumors, stratified by tumor distance to the peritoneum, n = 154. μm micrometers, PM peritoneal metastases.
D Kaplan Meier analysis for 3-year risk of peritoneal metastases of all patients without pT4b tumors, stratified by tumor distance to the peritoneum, n = 154. μm micrometers, PM peritoneal metastases. 
When comparing only 2 categories, patients with a distance of <0.1 mm had significantly higher rates of 3-year peritoneal metastases than patients with a distance ≥0.1 mm (Log Rank, p = 0.006, Fig. 4A). Subgroup analyses excluding patients with R1 resection, signet ring cell carcinoma, and pT4b also showed significant differences in 3-year peritoneal metastases rates between the two categories in all cases (Fig. 4B–D).
A Kaplan Meier analysis for 3-year risk of peritoneal metastases stratified by the measured relationship of tumor cells to the peritoneum in mm, n = 189. mm millimeters, PM peritoneal metastases.  B Kaplan Meier subgroup analysis for 3-year risk of peritoneal metastases in all patients with a R0 resection, stratified by tumor distance to the peritoneum in mm, n = 164. mm millimeters, PM peritoneal metastases.
B Kaplan Meier subgroup analysis for 3-year risk of peritoneal metastases in all patients with a R0 resection, stratified by tumor distance to the peritoneum in mm, n = 164. mm millimeters, PM peritoneal metastases.  C Kaplan Meier subgroup analysis for 3-year risk of peritoneal metastases in all patients without signet ring cell histology, stratified by tumor distance to the peritoneum in mm, n = 178. mm millimeters, PM peritoneal metastases.
C Kaplan Meier subgroup analysis for 3-year risk of peritoneal metastases in all patients without signet ring cell histology, stratified by tumor distance to the peritoneum in mm, n = 178. mm millimeters, PM peritoneal metastases.  D Kaplan Meier analysis for 3-year risk of peritoneal metastases in all patients without pT4b tumors, stratified by tumor distance to the peritoneum in mm, n = 154. mm millimeters, PM peritoneal metastases.
D Kaplan Meier analysis for 3-year risk of peritoneal metastases in all patients without pT4b tumors, stratified by tumor distance to the peritoneum in mm, n = 154. mm millimeters, PM peritoneal metastases. 
Discussion
This side study of the COLOPEC trial with detailed central histopathological revision of all primary colon cancers, measuring the shortest distance of tumor cells to the free peritoneal surface in µm, enabled objective stratification of patients according to the risk of developing peritoneal metastases. A cutoff value of 100 µm resulted in significantly different 3-year peritoneal metastasis rates, which seemed to become even more pronounced when excluding R1 resections. In patients with shortest distances beyond 100 µm between tumor cells and the free peritoneal surface, N-category did not influence the risk of peritoneal metastases, while only the N2 category seemed to result in an additive risk in patients with 0–99 µm. Full peritoneal penetration correlated with more aggressive pathology features such as signet ring cell tumor type, infiltrating tumor border configuration, and high tumor budding. Interestingly, the pT4b category was overrepresented in patients with the shortest distance of ≥100 µm. Although validation is needed, these findings suggest that subjective assessment of peritoneal involvement can be replaced by an objective measurement in µm.
We have demonstrated that when tumor cells are within 100 µm of the free peritoneal surface, patients have similar chance of developing peritoneal metastases as patients with tumor cells penetrating the peritoneum fully (growing clearly onto the peritoneal surface). On the other hand, when the distance of tumor cells to the peritoneal surface was 100–999 µm, the risk of developing peritoneal metastases was similar as for tumors with ≥1000 µm distance. These are important observations because there has been a trend towards classifying tumors with less than 1 mm (1000 µm) distance as pT4a, provided that certain fibroinflammatory and other reactive peritoneal changes are also present7,16. However, the present study indicates that only the subgroup with a shortest distance up to 100 µm among the 0–1000 µm range carries the main risk of developing peritoneal metastases. This implies that pT4a definitions based on 1 mm distance are likely to overestimate the number of cases with risk of developing peritoneal metastases. The presence or absence of various fibroinflammatory and reactive changes was not of relevance to the risk of developing peritoneal metastases (Suppl. Table 3). These changes were found in most cases and were, therefore, poorly discriminative. It deserves to be mentioned that peritoneal changes can still serve as an indicator to examine additional levels and sections.
A persistent problem in the staging of colorectal cancer retains the differentiation between pT3 and pT4a categories. The TNM-definition of pT4a lacks specificity on the topic with controversy regarding the exact criteria that should be used as summarized earlier8. While some pathologists stick to the criterion of full peritoneal penetration (extending onto the peritoneal surface), others consider pT4a in cases with cancer cells growing variably at, close to or within 1 mm of the peritoneal surface, especially in the presence of particular reactive changes7. Some authors have also proposed using the peritoneal elastic lamina as a reference line in differentiating between pT3 and pT4a19. Variation in national guidelines and literature proposals, all offering criteria that are bound by subjectivity, have resulted in interobserver variation as emphasized in three recent studies8,9,10. Measurement of the distance from the tumor cells to the free peritoneal surface, irrespective of the type of intervening stroma/tissue or peritoneal changes, could be an objective way of helping pathologists to diagnose pT4a more consistently. This can be easily performed digitally or using separate circular glasses with rulers for microscopes with 0.1 mm (=100 µm) intervals or built-in eyepieces, when digital assessment is not available. The reproducibility of sub-millimeter measurements when assessing peritoneal involvement needs to be further investigated, but it is likely that few practical difficulties arise, as measured at 0.1 mm intervals is already used for other purposes, for example for the distinction between micro-metastases and isolated tumor cells and for T-categorization of melanomas. Interestingly and in analogy to the present study, recent literature indicates that 0.1 mm cutoff value is more appropriate than 1.0 mm for differentiating between R0 and R1 for locally excised pT1 colorectal cancer26. Thus the importance of being able to perform sub-millimeter measurements in daily pathology practice seems to be increasing.
The higher risk of peritoneal metastases in patients with tumors showing full peritoneal penetration can be explained by the well-known theory of seeding of tumor cells within the peritoneal cavity after the peritoneal covering has been breached, subsequently attaching to the peritoneal surface elsewhere and progressing into peritoneal metastases. This risk of peritoneal metastases was 25% in the present study, which is similar to another study reporting on full penetration with a reported risk of 28%27. These data tell us that despite tumor cells having entered the peritoneal cavity, the efficiency of the metastatic process is overall not more than about 25–30%. This probably relates to the underlying tumor biology. These seeding theories, however, do not explain how tumors lacking full penetration (0–99 µm) also have a higher risk of peritoneal metastases. Some of these cases with a higher risk could be explained by inadequate sampling which has direct impact on the detection of pT4a, as shown previously8. In addition, handling of the specimen during surgical and pathological processes can wipe cells off the free peritoneal surface, only leaving subsurface growth. Also, discontinuous growth may play a role. Finally, reactive fibroinflammatory changes that occur after peritoneal tumor cell penetration has taken place may lead to the disappearance of the focus with full peritoneal penetration.
It is noteworthy that the risk of developing peritoneal metastases according to the shortest distance of tumor cells to the free peritoneal surface did not translate into differences in 3-year overall survival rates yet. The 5-year overall survival rates of the COLOPEC trial must be awaited to draw definitive conclusions, but the current data suggest that the distance-parameter, as proposed in this paper, might be mainly related to the risks of developing peritoneal metastases and less related to overall survival. As such, the question arises if the pT4a parameter is mainly meant to represent the risk of peritoneal metastases or more broadly overall survival. This is analogous to the question if the CRM status in rectal cancer is supposed to represent mainly the risk of local recurrence or overall survival? One way to solve this issue might be to subdivide pT3 into superficial and deep growth for predicting distal recurrences (via mainly lymphovascular spread) and reserve the pT4a parameter for mainly predicting peritoneal recurrences.
One of the advantages of the present study is the accurate detection of peritoneal metastases in the follow-up. Therefore, the frequency of peritoneal metastases in different categories may even be regarded as a reference rate for future research and quality control. The disadvantages retain possible selection bias related to the inclusion criteria of the COLOPEC trial, 3- instead of 5-year overall survival, and lack of performing multivariable analysis to adjust for possible confounders (which is the probable reason for pT4b not being prognostic on univariate analysis regarding peritoneal metastases). This was due to the relatively low patient and event sample. Therefore, future studies with larger patient cohorts are needed to confirm our findings. Despite multivariable analysis was not possible due to low number of events, we were able to perform informative subgroup analyses, excluding those patients who were possibly at high risk of the endpoint peritoneal metastases (R1 resection, signet ring cell tumor type).
In conclusion, we have provided objective means of classifying the depth of tumor invasion in relation to the peritoneal surface within a well-documented prospective trial with standardized follow-up including 18 months diagnostic laparoscopy, which can be used to stratify patients according to the risk of developing peritoneal metastases. Our results need verification in a larger study but may offer a basis to redefine the pT4a parameter using more objective criteria, regardless of any peritoneal changes.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jessup JM, GR, Asare EA, Benson III AB, Brierley JD, Chang GJ, et al. Colon and rectum. In: Amin MB, editor. 251–74 (Springer, 2017).
Lemmens, VE, Klaver, YL, Verwaal, VJ, Rutten, HJ, Coebergh, JW, de Hingh, IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer 128, 2717-2725 (2011).
van Gestel, YR, Thomassen, I, Lemmens, VE, Pruijt, JF, van Herk-Sukel, MP, Rutten, HJ, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol 40, 963–969 (2014).
Segelman, J, Granath, F, Holm, T, Machado, M, Mahteme, H, Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 99, 699–705 (2012).
Franko, J, Shi, Q, Meyers, JP, Maughan, TS, Adams, RA, Seymour, MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol 17, 1709–1719 (2016).
Yantiss, R. K. Persistent problems in colorectal cancer reporting. Surg Pathol Clin 10, 961–976 (2017).
Pantaleon Vasquez, R, Arslan, ME, Lee, H, King, TS, Dhall, D, Karamchandani, DM. T3 versus T4a staging challenges in deeply invasive colonic adenocarcinomas and correlation with clinical outcomes. Mod Pathol 34, 131–140 (2021).
Klaver, CEL, Bulkmans, N, Drillenburg, P, Grabsch, HI, van Grieken, NCT, Karrenbeld, A, et al. Interobserver, intraobserver, and interlaboratory variability in reporting pT4a colon cancer. Virchows Arch 476, 219–230 (2020).
Naso, J. R., Yang, H. M. & Schaeffer, D. F. Variability in synoptic reporting of colorectal cancer pT4a category and lymphovascular invasion. Arch Pathol Lab Med 145, 343–351 (2021).
Panarelli, NC, Hammer, STG, Lin, J, Gopal, P, Nalbantoglu, I, Zhou, L, et al. Reproducibility of AJCC criteria for classifying deeply invasive colon cancers is suboptimal for consistent cancer staging. Am J Surg Pathol 44, 1381–1388 (2020).
Manfredi, S, Bouvier, AM, Lepage, C, Hatem, C, Dancourt, V, Faivre, J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg 93, 1115–1122 (2006).
Li, X, An, B, Ma, J, He, B, Qi, J, Wang, W, et al. Prognostic value of the tumor size in resectable colorectal cancer with different primary locations: a retrospective study with the propensity score matching. J Cancer 10, 313–322 (2019).
Osterman, E. & Glimelius, B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire Swedish population. Dis Colon Rectum 61, 1016–1025 (2018).
Bori, R, Sejben, I, Svébis, M, Vajda, K, Markó, L, Pajkos, G, et al. Heterogeneity of pT3 colorectal carcinomas according to the depth of invasion. Pathol Oncol Res 15, 527–532 (2009).
Pollheimer, MJ, Kornprat, P, Pollheimer, VS, Lindtner, RA, Schlemmer, A, Rehak, P, et al. Clinical significance of pT sub-classification in surgical pathology of colorectal cancer. Int J Colorectal Dis 25, 187–196 (2010).
Panarelli, N. C., Schreiner, A. M., Brandt, S. M., Shepherd, N. A. & Yantiss, R. K. Histologic features and cytologic techniques that aid pathologic stage assessment of colonic adenocarcinoma. Am J Surg Pathol 37, 1252–1258 (2013).
Snaebjornsson, P, Coupe, VM, Jonasson, L, Meijer, GA, van Grieken, NC, Jonasson, JG. pT4 stage II and III colon cancers carry the worst prognosis in a nationwide survival analysis. Shepherd’s local peritoneal involvement revisited. Int J Cancer 135, 467–478 (2014).
Shepherd, N. A., Baxter, K. J. & Love, S. B. The prognostic importance of peritoneal involvement in colonic cancer: a prospective evaluation. Gastroenterology 112, 1096–1102 (1997).
Kojima, M., Yokota, M., Saito, N., Nomura, S. & Ochiai, A. Elastic laminal invasion in colon cancer: diagnostic utility and histological features. Front Oncol 2, 179 (2012).
Klaver, CEL, Wisselink, DD, Punt, CJA, Snaebjornsson, P, Crezee, J, Aalbers, AGJ, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol 4, 761–770 (2019).
Wöhlke, M., Schiffmann, L. & Prall, F. Aggressive colorectal carcinoma phenotypes of invasion can be assessed reproducibly and effectively predict poor survival: interobserver study and multivariate survival analysis of a prospectively collected series of 299 patients after potentially curative resections with long-term follow-up. Histopathology 59, 857–866 (2011).
Lugli, A, Kirsch, R, Ajioka, Y, Bosman, F, Cathomas, G, Dawson, H, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 30, 1299–1311 (2017).
van Pelt, GW, Kjær-Frifeldt, S, van Krieken, J, Al Dieri, R, Morreau, H, Tollenaar, R, et al. Scoring the tumor-stroma ratio in colon cancer: procedure and recommendations. Virchows Arch 473, 405–412 (2018).
Sobin, L. H. & Fleming, I. D. TNM classification of malignant tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 80, 1803–1804 (1997).
Quirke, P, Cuvelier, C, Ensari, A, Glimelius, B, Laurberg, S, Ortiz, H, et al. Evidence-based medicine: the time has come to set standards for staging. J Pathol 221, 357–360 (2010).
Gijsbers, KM, van der Schee, L, van Veen, T, van Berkel, AM, Boersma, F, Bronkhorst, CM, et al. Impact of ≥ 0.1-mm free resection margins on local intramural residual cancer after local excision of T1 colorectal cancer. Endosc Int Open 10, E282–e290 (2022).
Klaver, CEL, van Huijgevoort, NCM, de Buck van Overstraeten, A, Wolthuis, AM, Tanis, PJ, van der Bilt, JDW, et al. Locally advanced colorectal cancer: true peritoneal tumor penetration is associated with peritoneal metastases. Ann Surg Oncol 25, 212–220 (2018).
Acknowledgements
The authors would like to thank the NKI-AVL Core Facility Molecular Pathology and Biobanking (CFMPB), the Dutch National TissueArchive Portal (DNTP) and all involved pathology laboratories for help with retrieving the histological slide. The COLOPEC trial was funded by the Netherlands Organization for Health Research and Development (ZonMW) and the Dutch Cancer Society (KWF).
Author information
Authors and Affiliations
Consortia
Contributions
ESZ, DDW, CELK, JVDB, PJT, and PS performed study concept and design; ESZ, DDW, CELK, JVDB, PJT, and PS provided study data; ESZ, DDW, CELK, JVDB, PJT, and PS provided data analysis and interpretation of data; ESZ, DDW, CELK, JVDB, PJT, and PS have read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Approval of this study was obtained with the approval assessment process of the COLOPEC trial protocol by the institutional board of the Amsterdam UMC. Patients had to provide written informed consent to participate in the COLOPEC trial.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zwanenburg, E.S., Wisselink, D.D., Klaver, C.E.L. et al. The measured distance between tumor cells and the peritoneal surface predicts the risk of peritoneal metastases and offers an objective means to differentiate between pT3 and pT4a colon cancer. Mod Pathol 35, 1991–2001 (2022). https://doi.org/10.1038/s41379-022-01154-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01154-z