Abstract
We aimed to detect the clinicopathological features and immune microenvironment of double-hit/triple-hit lymphoma in the gastrointestinal tract (GI-DHL/THL) and identify the best diagnostic strategies. A total of 114 cases, including 15 GI-DHL/THL, 42 non-GI-DHL/THL and 57 control diffuse large B-cell lymphoma (DLBCL) cases, were comparatively analyzed for their clinicopathological characteristics, the expression of the immune-regulatory checkpoint PD-L1 and immune microenvironment. We applied univariate and multivariate analyses to determine predictors of DHL/THL. GI-DHL/THL patients showed a higher prevalence of previous infection with hepatitis B virus (HBV) than those with GI-DLBCL. Morphologically, 87% of cases exhibited features of DLBCL. Regarding immunohistochemistry results, the MYC protein expression and the Ki-67 proliferation index were significantly higher in the GI-DHL/THL group than in the GI-DLBCL group. The main source of PD-L1 expression in DHL was tumor-associated macrophages, whereas some tumor cells were positive for PD-L1 in GI-DLBCL cases, as determined through multiplex immunofluorescence staining. The multivariable logistic analysis suggested that 5 variables, namely, age, Mum1, CD10, MYC, and HBV infection status, reflect the risk of DHL/THL. The GI-DHL/THL group show different clinicopathological features and immune microenvironments from DLBCL, which might suggest that different signaling pathways are involved. More work is needed to elucidate the pathogenic mechanism of GI-DHL/THL.
Similar content being viewed by others
Introduction
The gastrointestinal (GI) tract is the most common extranodal site involved in non-Hodgkin lymphoma (NHL)1. Diffuse large B-cell lymphoma (DLBCL) and marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) constitute the largest parts of GI-NHL. Similar to DLBCL in other sites, GI-DLBCL is a heterogeneous group including DLBCLs that have transformed from low-grade B cell lymphoma as well as de novo disease. The latter can be further subdivided into DLBCL, not otherwise specified (NOS), double-hit/triple-hit lymphoma (DHL/THL) and high-grade B-cell lymphoma (HGBL), NOS and other types since 2016 by the application of the gold standard test fluorescence in situ hybridization (FISH). Double-hit/triple-hit lymphoma is defined as HGBL with MYC and BCL2 or/and BCL6 rearrangements2. Clinically, DHL/THL is characterized by a rapidly progressive and advanced stage of disease, high rates of central nervous system (CNS) involvement, refractoriness to conventional therapy and inferior clinical outcomes2,3.
Double-hit/triple-hit lymphoma remains a rare type of lymphoma. The overall incidence varies according to reports and makes up 6–14% of DLBCL cases4,5,6. The diagnosis and treatment of DHL/THL can still be challenging, although much progress has been made in various aspects such as molecular genetic studies and treatment in recent years. The molecular genetic studies included whole-genome sequencing, copy-number variants, and structural variants7,8. Double-hit/triple-hit lymphoma has a spectrum of morphology, for example, some appear similar to DLBCL or Burkitt lymphoma (BL), and some have blastoid or lymphoblastic morphology or gray-zone features. Most DHL/THLs are the germinal center B cell-like (GCB) phenotype as classified by the Hans algorithm, that is, CD10 and BCL6 expression are found in most of these lymphomas (75-90%)2. The differential diagnosis between DHL and DLBCL cannot be made without knowledge of the MYC, BCL2, and BCL6 rearrangement status. A subset of DHL/THL will likely be missed regardless of the strategy pathologists use to perform FISH testing unless they order tests for every case. The availability of FISH varies significantly from country to country due to limited diagnostic laboratory resources, biopsy tissue, and expensive price6,9,10. The situation may be worse in developing countries because of the high cost of FISH testing. In addition, other lymphoid and hematological neoplasm mimics, such as transformed follicular lymphoma (FL), which may have both MYC and BCL2 rearrangements and lymphoblastic lymphoma, must be excluded because similar genetic abnormalities can also be detected in these lymphomas. Regarding treatment, no standard therapeutic approach has been recommended. DA-R-EPOCH, BL regimens and R-CHOP are the most common regimens used for treating DHL5,9.
Although DHL/THL is as common in the GI tract as DLBCL11, no detailed description has been reported to date. Thus, the present study aimed to characterize the clinicopathological features of these lymphomas by comparatively analyzing a series of GI-DHL/THL, non-GI-DHL/THL and GI-DLBCL cases. The PD1/PD-L1 pathway is critical in immunotherapy of malignancies including certain subtypes of lymphoma because it has been shown to be an important immune checkpoint in recent years. Most lymphomas show low efficacy of immune checkpoint inhibitor monotherapy, but the underlying reasons remain unclear12,13. In the present study, PD-L1 expression and the immune microenvironment were explored in an attempt to better understand their role in immunotherapy. In addition, we attempted to develop a comprehensive model combining immunohistochemistry (IHC) signatures and clinical features to predict the risk of DHL/THL in patients with DLBCL, providing a useful tool for pathologists.
Materials and methods
Study population
Fifty-seven cases of DHL/THL and 57 control cases of DLBCL were retrospectively collected from 3 institutions (Department of Pathology of Sun Yat-sen University Cancer Center, Zhongnan Hospital of Wuhan University, Xijing Hospital and School of Basic Medicine). Specifically, the 57 cases in the DHL/THL cohort consisted of 15 cases of GI-DHL/THL, 21 cases of lymph node DHL/THL and 21 cases of extranodal DHL/THL. Fifty-one of these cases were included in our previous publication11. The inclusion criteria for control DLBCL cases were those who underwent a FISH study at diagnosis and showed no MYC rearrangement from March 2016 to September 2019. For comparison, 57 control cases of DLBCL were consecutive patients who met the inclusion criteria and randomly matched with DHL/THL group according to the anatomic site (GI, LN, and non-GI / LN). The 57 DLBCL cases in the control cohort consisted of 15 cases of GI-DLBCL and 42 cases of DLBCL in non-GI locations (Table 1). The present study was approved by the Research Ethics Committee of these 3 institutes. Clinical data were retrospectively searched from the electronic medical records, including age, sex, tumor site, clinical features, initial presentation, imaging data, Ann Arbor stage, serum lactate dehydrogenase (LDH) levels, hepatitis B virus (HBV) infection status and other laboratory results from the initial examination for definite diagnosis of DHL/THL11.
Pathology review and ancillary testing
The tissue samples were formalin-fixed and paraffin-embedded (FFPE) specimens from either biopsies or resections. All cases underwent primary review by two hematopathologists in each institute and secondary review by Drs. Rao and Tian. Ancillary testing included IHC staining, in situ hybridization (ISH) and FISH, which were performed according to previously published methods11. The antibodies used for IHC are shown in Supplementary Table S2. The tumor cell-of-origin (COO) GCB and non-GCB subtypes were distinguished for CD10, BCL6, and MUM1 expression using thresholds of ≥30% to define positivity. Tumors were defined as double-expressor lymphoma (DEL) when MYC and BCL2 protein expression was present in at least 40% and 50% of tumor cells, respectively14. All 114 cases were tested for MYC, BCL2, and BCL6 rearrangements. The probe for MYC (8q24) rearrangement was a break-apart probe (01N63-020, Vysis; Abbott Laboratories, IL). The probe for BCL6 (3q27) rearrangement was also a break-apart probe (01N23-020, Vysis; Abbott Laboratories). In addition, the IGH/BCL2 fusion translocation t (14; 18) probe (08L60-020, Vysis; Abbott Laboratories) was used for the test of BCL2. FISH test was performed according to the manufacturer’s instructions.
PD-L1 expression and the immune microenvironment of DHL and DLBCL
Rabbit monoclonal anti-PD-L1 antibody was obtained from Cell Signaling Technology (clone E1L3N, dilution 1:200; Cell Signaling Technology, Danvers, MA, USA). There has been no consensus on the positive threshold of PD-L1 in lymphoma. We defined score 1 as positive staining in ≤5% of tumor cells and score 2 as positive staining in >5% of tumor cells in the current study15,16. At the same time, the intensity of immunohistochemistry was graded as negative, weak, intermediate or high intensity.
Manual multiplex immunofluorescence (mIF) staining was performed in 4-μm sections obtained from FFPE blocks by using the Opal 7-Color IHC Kit (PerkinElmer, Waltham, MA). The stained slides were scanned with a Vectra multispectral microscope (PerkinElmer)17. Prediluted, fluorophore-conjugated antibodies against PD-L1 (same as above), CD20 (from Leica), CD4 (DAKO), CD8 (DAKO), MYC (DAKO) and CD68 (PG-M1, DAKO) were used. Human tonsil FFPE tissues were used with and without primary antibodies as positive and negative (autofluorescence) controls, respectively. The procedure was performed as described in a previously published reference17.
Follow-up
Overall survival was defined as the period from the day of diagnosis to death or the last follow-up. We extended the follow-up period to August 2021 for patients who were alive at the last follow-up in 2019, as described in the previous publication11.
Statistical analysis
Normally distributed data are summarized as the mean (range). The median (range) is presented for non-normally distributed data. The Mann–Whitney U test or Student’s t test was used to compare continuous variables. Categorical variables were expressed as frequencies and were compared using Chi-squared analysis, Fisher’s exact test, Yates’ continuity-corrected chi-square test or the Mann–Whitney U test. Overall survival was analyzed by the Kaplan–Meier method, and comparisons were performed with the log-rank test. Univariate and multivariate logistic regression analyses were performed to determine predictors of DHL/THL. We randomly divided the entire dataset into a training dataset (70% of the original samples) and a validation dataset (30% of the original samples). Missing values were imputed based on the means. All variables with p values less than 0.05 in univariate analysis were selected for multivariate analysis. Backward feature selection was performed with the Akaike information criterion. The variables selected by the above procedure were applied to develop the final model on the training dataset. The discriminative ability of the model was measured by area under receiver operating characteristic curve (ROC, R package pROC), and the optimal cutoff with sensitivity of 80% was calculated. In addition, the discrimination of the model was internally validated by applying the model to validation dataset without any adjustment. The results of the modeling process were also converted to a nomogram, which depicted the relative importance of each predictor and could be used to calculate the estimated value of DHL/THL risk. We used R statistical software (version 4.0.1) to complete the analysis. Statistical analysis was performed with SPSS 22.0 software to analyze the clinical data. A p value <0.05 was considered statistically significant.
Results
Clinical characteristics and pathological findings of GI-DHL/THL
The demographic and clinical information of GI-DHL/THL patients and the morphological features of their tumors are summarized in Table 2. There were 9 males and 6 females in this group. The median age of the patients was 52.9 years (ranging from 20 to 82 years). The stomach was the most common site of GI-DHL/THL (8/15, 53%), followed by the small intestine (3/15, 20%), colon (3/15, 20%) and rectum (1/15, 7%). The main manifestations at onset of disease included abdominal pain, bloating, vomiting, abdominal mass, melena, and frequent and loose stools. Six patients (40%) had a past history of previous infection with HBV. Ten of the patients (75%) presented with Ann Arbor stage III/IV. No CNS or bone marrow involvement was identified. The International Prognostic Index (IPI) scores of nearly half of the cases (7/15) were above 3 (Table 3). They were mainly treated with R-CHOP or DA-R-EPOCH as frontline immunochemotherapy.
The cohort contained 14 cases of DHL and 1 case of THL, and the prevalence of MYC/BCL6 DHL (8/15, 53.3%) was higher than that of MYC/BCL2 DHL (6/15, 40%) (Table 2). The only case of THL was located in the gastric angle of a 59-year-old female. Morphologically, 13 cases (87%) exhibited features of DLBCL, while the other 2 had the appearance of unclassifiable B-cell lymphoma, with features intermediate between DLBCL and BL. Regarding IHC staining results (Tables 3), 73.3% of the cases (11/15) were classified as belonging to the GCB subtype, and DEL constituted half of the cases (7/13, 53.8%; MYC IHC was not available in 2 cases). The median MYC protein expression was 70%. The average Ki-67 proliferation index was 90%, ranging from 60% to 98%.
As of August 2021, six patients had died of the disease, and seven in the cohort were still alive. The other two were lost to follow-up. The survival time ranged from 1 to 43 months, the latter being the full length of the follow-up period.
Parallel comparison between GI-DHL/THL and non-GI-DHL/THL or other extranodal DHL/THL cases
There was no significant difference between GI-DHL/THL and overall non-GI DHL/THL (n = 42), GI-DHL/THL and exclusively nodal DHL/THL (n = 21), or GI-DHL/THL and other extranodal DHL/THL (n = 21) (not shown) in clinical characteristics, such as age, sex, B symptoms, stage, CNS or bone marrow involvement, serum LDH level, and IPI score (Table 3). As for pathological parameters, there were no prominent differences, such as morphology, COO, rates of DEL, expression of selected immunomarkers such as the MYC protein, Ki-67 index, or EBER status. MYC/BCL6 DHL constituted a larger percentage than MYC/BCL2 DHL in all 3 groups, but there was no significant difference between groups.
Comparison of clinicopathological features and immunophenotypic differences between GI-DHL/THL and GI-DLBCL cases
GI-DHL/THL patients were found to be younger than GI-DLBCL patients (Table 3). More patients (10/13, 76.9%) presented with Ann Arbor stage III/IV disease in the GI-DHL/THL group than in the GI-DLBCL group (4/15, 26.7%) (p < 0.05). The rate of previous HBV infections was significantly higher in the GI-DHL/THL group than in the GI-DLBCL group. There were no prominent differences in other clinical parameters, such as sex, B symptoms, CNS or bone marrow involvement, serum LDH level, and IPI score (Table 3). There was also no significant difference in morphology, COO, rate of DEL or EBER status. However, the expression of the MYC protein and the Ki-67 proliferation index were significantly higher in the GI-DHL/THL group than in the GI-DLBCL group (p < 0.05). No other parameters or immunomarkers showed differential expression between these two groups.
PD-L1 and mIF results in DHL/THL and DLBCL cases
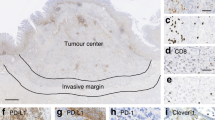
Fifteen tissue samples from DHL (representing 10 GI and 5 non-GI cases) and 57 tissue samples from DLBCL were available for IHC of PD-L1. The intensity and extent of expression differed between DHL/THL and DLBCL cases, but only the extent was significantly different between these two groups (p < 0.05) (Table 4). DLBCLs mainly showed a weak to moderate PD-L1 staining pattern (Table 4 and Fig. 1A, B), and 50 cases (87.7%) showed over 5% positive tumor cells, whereas DHLs demonstrated intermediate intensity but sparse staining (<5% of tumor cells) (Table 4 and Fig. 1G, H). That is, more lymphoma cells in DLBCL cases showed PD-L1 positivity, while hardly any PD-L1-expressing tumor cells were identified in DHL cases. Consistent with the IHC results, mIF showed that coexpression of CD20 and PD-L1 was more common in DLBCL (Fig. 1 C, D, F and I, J, L). Coexpression of PD-L1 and CD20 was less frequently observed in DHL, while cells positive for both PD-L1 and CD68 were observed, which demonstrated that PD-L1 was mainly expressed in macrophages in DHL (Fig. 1D–F and J–L). Seldom were cells positive for PD-L1 and CD4 or CD8 (not shown). Interestingly, one DLBCL was incidentally found to have strong and intense expression of CD4 in the mIF image (not shown).
Regarding the microenvironment, coexpression of CD20, PD-L1 and CD68 were detected by multiplex immunofluorescence. A, G: H&E staining; B, H: PD-L1 expression (IHC); C, I: CD20 (green); D, J: PD-L1 (red); E, K: CD68 (yellow); F, L: merge image for CD20, PD-L1 and CD68 (original magnification of all images ×400).
DHL/THL screening strategy
The overall DHL/THL cohort, consisting of 57 cases, showed a younger age at the onset of disease, a higher incidence of previous HBV infection, and higher percentages of the GCB type and DEL phenotype than the cohort of 57 DLBCL cases (Table 5). In accordance with the more common GCB phenotype, higher expression of CD10 and lower expression of Mum-1 were observed in the DHL/THL cohort. MYC and the Ki-67 proliferation index were significantly different between these two groups, that is, the DHL/THL cohort had higher MYC protein expression and faster growth. During the follow-up period, the proportion of patients died of the disease in the DHL/THL group was significantly higher than that in the DLBCL group (p < 0.05). There were no differences in clinical characteristics, MYC IHC, or the Ki-67 proliferation index between DHL/THL and DLBCL of the GCB type (not shown).
The final logistic model was developed from the 5 variables selected using backward feature selection. The formula was as follows: risk score = 0.03 − 0.05*Age − 1.65 *Mum1 + 0.20*CD10 + 0.06*MYC + 1.61*HBV infection status; the results are shown in Supplementary Table S1. The categorical variables in the formula were defined as follows: Mum1 IHC (0 = negative, 1 = positive); CD10 IHC (0 = negative, 1 = positive); HBV infection status (0 = no history of HBV infection, 1 = previous HBV infection). The area under the curve (AUC) was 0.89 (95% confidence interval: 0.81–0.96) in the training dataset (Fig. 2A). The optimal cutoff was 0.47, which suggested that DLBCL patients with risk scores greater than 0.47 were more likely to have DHL/THL. The sensitivity was 0.81, and the specificity was 0.77. Applying the model to the validation dataset, we obtained an AUC of 0.85 (95% confidence interval: 0.72–0.99) (Fig. 2B). With the same cutoff, the sensitivity and specificity in the validation dataset were 0.86 and 0.77, respectively. According to the nomogram (Fig. 2C), the contributions of all variables could be simply added, and the results reflected the individual risk of DHL/THL, which provides a straightforward model for pathologists to decide whether to perform FISH testing on patients with DLBCL. We have used GI-DHL/THL and GI-DLBCL to verify the model, and obtained an AUC of 0.982, which showed that the model could also be applied to GI-DHL/THL cases (Supplementary Fig. 1).
Progression and survival
The prognosis of patients with GI-DHL/THL was worse than that of patients with GI-DLBCL (Fig. 3A). In addition, the overall survival of the DHL/THL group was also worse than that in the DLBCL group (Fig. 3B), in line with the follow-up mentioned above. Survival analysis showed no difference between GI-DHL/THL and non-GI-DHL/THL or among DHL/THL in GI, nodal and other extranodal sites (not shown).
A Comparison of the prognosis of patients with GI-DHL/THL and GI-DLBCL. Two of 15 patients in GI-DHL/THL group were lost to follow-up. B Comparison of the prognosis of patients with overall DHL/THLs and DLBCLs. Three of 57 patients in DHL/THL group were lost to follow-up, including 2 cases from GI and 1 case from lymph nodes.
Discussion
Thirty to forty percent of extranodal NHLs occur in the GI tract1. Additionally, the GI tract is the most common extranodal site of DLBCL2. By whole-exome sequencing, the genomic mutation profile of GI-DLBCL has been found to differ from that of other DLBCLs in that the former has higher mutation frequencies in TP53, MUC16, CCND3, HIST1H1C and ID3 but lower mutation frequencies in MYD88, CREBBP, BCL2, KMT2D, PIM1 and EZH218. Importantly, the GI tract is also one of the most common sites of DHL/THL11. Interestingly, there was no CNS or bone marrow involvement in the present GI-DHL/THL cases, which is inconsistent with previous reports2,3. A larger sample size and longer follow-up will be needed to test the possible implication that GI-DHL/THL is slightly more indolent or less aggressive than DHL/THL in other sites. It was found that the prevalence of previous HBV infection was higher in the GI-DHL/THL group than in the GI-DLBCL group; thus, previous HBV infection may be a risk factor for GI-DHL/THL. This is consistent with a previous publication and the results of the final logistic analysis in the present study11. No other clinical parameter showed a significant difference between GI-DHL/THL and non-GI-DHL/THL or GI-DLBCL.
Morphologically, 13 GI-DHL/THL cases (87%) exhibited features of DLBCL, while the other 2 had the appearance of unclassifiable B cell lymphoma, with features intermediate between DLBCL and BL in the current study. The differential diagnoses of GI-DHL/THL might include, but are not limited to, poorly differentiated carcinoma, neuroendocrine carcinoma and other aggressive B cell lymphomas. The most important differential diagnoses might be DLBCL-NOS, MYC single-hit DLBCL, and transformed double-hit/triple-hit follicular lymphoma. However, differential diagnosis is not challenging when the past medical history, observation of the follicular dendritic cell (FDC) meshwork, and the performance of additional FISH testing for BCL2 and BCL6 are utilized. From the present study results, a high Ki-67 proliferation index and MYC expression are helpful to prompt testing for MYC rearrangement. In our study, MYC/BCL6 rearrangement was more common than MYC/BCL2 rearrangement in the GI tract, as in other sites.
Immune checkpoint inhibitors, such as PD1/PD-L1 inhibitors, have been reported since 2018 to be involved in various tumors, including classical Hodgkin lymphoma. The prognostic value of PD-L1 in the treatment of DLBCL remains to be verified because of conflicting results reported in the literature. In the present study, by means of IHC and mIF, we found that PD-L1 is mainly expressed by tumor cells and tumor‐associated macrophages (TAMs) in DLBCL, whereas TAMs are the main source of PD-L1 expression in DHL/THL, suggesting that DHL has a different microenvironment from DLBCL. Previous findings indicated that PD-L1 is predominantly expressed in TAMs but not lymphoma B cells based on dual IHC staining19. This is slightly different from our findings. Few T cells have been found either in GI-DLBCL or DHL/THL. The result of mIF was consistent with the result of IHC but provided us with a more straightforward way to verify the spatial information and microenvironment of the tumor cells. The present study suggests that DHL/THL is a “colder” tumor than DLBCL, which is in line with the fact that DHL/THL needs stronger therapeutic regimens and has a worse prognosis than DLBCL. In addition, TAM-associated targeted treatment may be helpful for DHL/THL in the near future because TAMs outnumber other immune cells. Because of the small sample size, we could not stratify the cases for further investigation.
Although FISH is a standard technique for testing DHL/THL, its availability has some restrictions due to financial issues, tissue quantity, and the resources of the diagnostic laboratory. In addition, there is the possibility of missing those DHL/THL tumors with cryptic mutations or only copy number alterations7,20. For instance, one study identified a unique set of alternative DHL/THL cases that feature concurrent translocations and copy number gains in MYC, BCL2, and/or BCL6, but this special entity cannot readily be identified using standard FISH alone20. A recommended method is to test for MYC rearrangement in all tumors with DLBCL morphology and then test for BCL2 and BCL6 rearrangements in the cases where FISH detects MYC rearrangement6. However, a new alternative screening strategy is still necessary. LMO2 loss may be a good predictor of the presence of MYC translocation in CD10-positive DLBCL21. However, we could not find a correlation between the LMO2 IHC signal and rearrangement in DHL/THL (Supplementary Table S3).
There has been considerable controversy over what subset of patients with DLBCL might have DHL/THL in the absence of FISH results. Screening strategies such as MYC IHC or COO plus DEL miss approximately one-quarter to one-third of DHL/THL cases6,8. Therefore, comprehensive screening strategies are urgently needed. We developed a comprehensive model with two types of independent predictors (IHC signatures and clinical features) using the following variables: age, Mum1, CD10, MYC, and HBV infection status. Furthermore, a nomogram was proposed to provide pathologists with a visual tool with the candidate predictors.
In the IPI scoring system, age over 60 years old is a prognostic risk factor. In this study, the age of onset of DHL/THL was younger than that of DLBCL, which suggested that early onset might indicate more aggressive biological behavior.
It is known that CD10 and BCL6 are considered as markers of the GCB phenotype and MUM1 is the marker of the non-GCB phenotype per the Hans classification22. Consistent with a previous publication23, we also found that a great majority of DHL/THL cases are of the GCB subtype. In the present study, the percentage of CD10 positive cases in the DHL/THL group was significantly higher than that in the DLBCL group. Meanwhile, the percentage of MUM1 positive cases in the DHL/THL group was significantly lower than that in the DLBCL group. Other studies have shown that almost all cases of MYC/BCL2 DHL have the GCB phenotype, while MYC/BCL6 DHL has a lower frequency of the GCB phenotype24. Therefore, if only DLBCLs with the GCB type are checked by FISH testing, a subset of DHL/THL will be missed, especially MYC/BCL6 DHL. Abdullah et al. reported that MYC IHC was a predictor for DHL/THL25. MYC IHC was also found to be a risk factor in our model. Hepatitis B surface antigen (HBsAg)-seropositive patients have a significant two- to threefold increase in their risk of developing B cell NHL compared with HBsAg-seronegative patients, and the increase is especially large for DLBCL26. Several lines of investigation have shown that HBV infection status is a risk factor for NHL and influences the choice of immunochemotherapy drugs27,28. We found that the HBV infection rate of DHL/THL patients was significantly higher than that of DLBCL patients, which indicated that HBV might be an important risk factor affecting the tumorigenesis of DHL/THLs. Ha Nguyen et al. validated a novel gene expression assay (DLBCL90) to distinguish DHL/THL containing a BCL2 translocation, which requires sequencing data29. Interestingly, all variables in our models were easily obtained, which means that it is more practical than the DLBCL90 assay. We proposed the model based on multivariate logistic regression analysis of selected variables. The ROC of the validation dataset had an AUC of 0.85, and that of the training dataset had an AUC of 0.89, which supported the ability of the model to detect DHL/THL. We believe the model will help to identify patients with DLBCL who require FISH testing and reduce unnecessary testing. This model can also be applied to DHL/THL cases of different locations and was verified with GI-DHL/THLs in the present study.
The present study has several limitations. First, the sample size was small, and potential selection bias could not be excluded, although there have been no previous reports of GI-DHL/THL. Second, the majority of the GI-DHL/THL samples were obtained from biopsies, and we were therefore unable to perform further molecular testing such as next-generation sequencing. More work is needed to elucidate the pathogenic mechanism resulting in this special entity.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Olszewska-Szopa M, Wrobel T. Gastrointestinal non-Hodgkin lymphomas. Adv Clin Exp Med 28, 1119–1124 (2019).
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th Edition ed. 69372 Lyon Cedex 08, France: International Agency for Research on Cancer (IARC) 585; (2017).
Rosenthal A. Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev 31, 37–42 (2017).
Li M, Zhang QL, Zhao W, Huang X, Gong LP, Shi QF, et al. The incidence of high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements in diffuse large B-cell lymphoma. Zhonghua Xue Ye Xue Za Zhi 42, 124–128 (2021).
Li S, Lin P, Medeiros LJ. Advances in pathological understanding of high-grade B cell lymphomas. Expert Rev Hematol 11, 637–648 (2018).
Scott DW, King RL, Staiger AM, Ben-Neriah S, Jiang A, Horn H, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood 131, 2060–2064 (2018).
Hilton LK, Tang J, Ben-Neriah S, Alcaide M, Jiang A, Grande BM et al. The double-hit signature identifies double-hit diffuse large B-cell lymphoma with genetic events cryptic to FISH. Blood 134, 1528–1532 (2019).
Collinge B, Ben-Neriah S, Chong L, Boyle M, Jiang A, Miyata-Takata T, et al. The impact of MYC and BCL2 structural variants in tumors of DLBCL morphology and mechanisms of false-negative MYC IHC. Blood 137, 2196–2208 (2021).
Aurer I, Dreyling M, Federico M, Tilly H, Linton K, Kimby E, et al. Double hit lymphoma diagnosis and treatment in Europe-a cross-sectional survey of clinical practice by the EHA Lymphoma Working Party (EHA LyG). Hemasphere 4, e481 (2020).
Copie-Bergman C. Double-hit DLBCL: should we limit FISH testing? Blood 131, 1997–1998 (2018).
Zhang J, Weng Z, Huang Y, Li M, Wang F, Wang Y, et al. High-grade B-cell lymphoma with MYC, BCL2, and/or BCL6 translocations/rearrangements: clinicopathologic features of 51 cases in a single institution of South China. Am J Surg Pathol 44, 1602–1611 (2020).
Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharm 12, 731798 (2021).
Hatic H, Sampat D, Goyal G. Immune checkpoint inhibitors in lymphoma: challenges and opportunities. Ann Transl Med 9, 1037 (2021).
Dodero A, Guidetti A, Marino F, Tucci A, Barretta F, Re A, et al. Dose-adjusted EPOCH and rituximab for the treatment of double expressor and double-hit diffuse large B-cell lymphoma: impact of TP53 mutations on clinical outcome. Haematologica 107, 1153–1162 (2022).
Fisher KE, Ferguson LS, Coffey AM, Merritt BY, Curry JL, Marcogliese AN, et al. Programmed cell death ligand 1 expression in aggressive pediatric non-Hodgkin lymphomas: frequency, genetic mechanisms, and clinical significance. Haematologica 107, 1880–1890 (2022).
Wang J, Shang S, Li J, Deng H, Ouyang L, Xie H, et al. PD-L1 and miR-34a are prognostic factors for primary gastric diffuse large b-cell lymphoma patients treated with R-CHOP. Cancer Manag Res 12, 4999–5008 (2020).
Guo CY, Zhu Q, Tou FF, Wen XM, Kuang YK, Hu H. The prognostic value of PKM2 and its correlation with tumour cell PD-L1 in lung adenocarcinoma. BMC Cancer 19, 289 (2019).
Li P, Chai J, Chen Z, Liu Y, Wei J, Liu Y, et al. Genomic mutation profile of primary gastrointestinal diffuse large B-cell lymphoma. Front Oncol 11, 622648 (2021).
Breinholt MF, Oliveira D, Klausen TW, Gang AO, Schejbel L, Pedersen MO, et al. High-grade B-cell lymphomas with MYC and BCL2 translocations lack tumor-associated macrophages and PD-L1 expression: A possible noninflamed subgroup. Hematol Oncol 39, 284–292 (2021).
Krull JE, Wenzl K, Hartert KT, Manske MK, Sarangi V, Maurer MJ, et al. Somatic copy number gains in MYC, BCL2, and BCL6 identifies a subset of aggressive alternative-DH/TH DLBCL patients. Blood Cancer J 10, 117 (2020).
Colomo L, Vazquez I, Papaleo N, Espinet B, Ferrer A, Franco C, et al. LMO2-negative expression predicts the presence of MYC translocations in aggressive B-cell lymphomas. Am J Surg Pathol 41, 877–886 (2017).
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103, 275–282 (2004).
Ganapathi KA, Brown LE, Prakash S, Bhargava P. New developments in non-Hodgkin lymphoid malignancies. Pathology 53, 349–366 (2021).
Li S, Desai P, Lin P, Yin CC, Tang G, Wang XJ, et al. MYC/BCL6 double-hit lymphoma (DHL): a tumour associated with an aggressive clinical course and poor prognosis. Histopathology 68, 1090–1098 (2016).
Alsuwaidan A, Koduru P, Fuda F, Manuel Jaso J, Chen M, Rosado F, et al. A combined biomarker of bright CD38 and MYC ≥55% is highly predictive of double-/triple-hit high-grade B-cell lymphoma. Am J Clin Pathol 158, 338–344 (2022).
Zhou X, Pan H, Yang P, Ye P, Cao H, Zhou H. Both chronic HBV infection and naturally acquired HBV immunity confer increased risks of B-cell non-Hodgkin lymphoma. BMC Cancer 19, 477 (2019).
Taborelli M, Polesel J, Montella M, Libra M, Tedeschi R, Battiston M, et al. Hepatitis B and C viruses and risk of non-Hodgkin lymphoma: a case-control study in Italy. Infect Agent Cancer 11, 27 (2016).
Kusumoto S, Arcaini L, Hong X, Jin J, Kim WS, Kwong YL, et al. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood 133, 137–146 (2019).
Nguyen H, Perry A, Skrabek P, Nasr M, Herrera AF, Bedell V, et al. Validation of the double-hit gene expression signature (DLBCL90) in an independent cohort of patients with diffuse large B-cell lymphoma of germinal center origin. J Mol Diagn 23, 658–664 (2021).
Acknowledgements
The authors appreciate the assistance of Dr. Jason X. Cheng, MD, PhD, of Department of Pathology, University of Chicago Medical Center, USA. This work was supported by Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund with project No. znpy2019072.
Author information
Authors and Affiliations
Contributions
ST and HR designed the research; JG, JX and YC performed the experiments; XX, HC and JZ assisted with the experiments; JG and YC analyzed the results and made the tables and figures; ST, ZW and HR reviewed the cases and ST wrote the paper. This manuscript was approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee of Zhongnan Hospital of Wuhan University, Sun Yat-sen University Cancer Center and Xijing Hospital and performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, J., Cai, Y., Wang, Z. et al. Double/triple hit lymphoma in the gastrointestinal tract: clinicopathological features, PD-L1 expression and screening strategy. Mod Pathol 35, 1667–1676 (2022). https://doi.org/10.1038/s41379-022-01150-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01150-3