Abstract
Central nervous system (CNS) germ cell tumors (GCTs) represent 2–3% of all primary CNS tumors. The majority are germinomas, which are radiosensitive and have an excellent prognosis. Contrarily, CNS non-germinomatous GCTs (NGGCTs) have less favorable prognosis and require more aggressive treatment. The expression of checkpoint/immune markers in CNS GCTs, particularly NGGCTs, is unknown. We previously reported a case of a patient whose intracranial NGGCT (predominantly choriocarcinoma) responded to immune checkpoint inhibition therapy. This case led us to evaluate our archive of intracranial GCTs for expression of PD-L1 and PD-1. With IRB approval, we searched the pathology archives at our institution for CNS GCTs. Demographic, radiologic, clinical, and histologic information was extracted from the medical records. Immunohistochemistry for lymphocytic markers (CD4, CD8, CD20), PD-1, and PD-L1 was performed. PD-L1 was considered positive if greater than 1% of tumor cells were positive and PD-1 was reported as a percentage of positive inflammatory cells. Fifty cases were identified, including 28 germinomas (mean age at diagnosis: 15.5 years; 17 males, 11 females), and 22 NGGCTs (mean age at diagnosis: 12.0 years, 21 males, 1 female). Germinomas were mostly suprasellar (17/28) and NGGCTs were predominantly pineal (17/22). Twenty-two germinomas (79%) were positive for PD-L1 expression, and 13 NGGCTs (57%) were positive for PD-L1. Cases of choriocarcinoma showed the most diffuse PD-L1 expression. PD-1 expression was seen in lymphocytes among 27/28 of the germinomas and 20/23 of the NGGCTs (ranging from 1–40% of lymphocytes). As expected, larger quantities of inflammatory cells were present in cases of germinoma. We demonstrate immune activity in CNS GCTs, and our results suggest that immune checkpoint inhibitors may be efficacious in the treatment of intracranial GCTs. Among NGGCTs, cases of choriocarcinoma showed the highest expression of PD-L1 in tumor cells, suggesting that this subtype may have the greatest benefit from checkpoint blockade.
Similar content being viewed by others
Introduction
Germ cell tumors (GCT) of the central nervous system (CNS) are a rare class of tumors with an overall incidence of 0.1 per 100,000 people per year1. These tumors predominantly affect males and have a peak incidence in patients between the ages of 10–14 years2. The CNS is the second most common site for extra-gonadal GCTs, and, in patients aged 0–19 years, GCTs comprise ~3–5% of primary CNS tumors in the US3. These tumors are generally divided into germinomas, the most common type of GCT, and non-germinomatous GCTs (NGGCT)4. The latter includes teratoma, choriocarcinoma, yolk sac tumor, and embryonal carcinoma. Germinomas have a good prognosis due to their sensitivity to chemotherapy and radiation, with a 10-year overall survival of over 90%5,6. However, NGGCTs have a 5-year survival rate ranging from 40–70% and require more aggressive treatment including aggressive chemotherapy and radiation therapy, with/without the addition of surgery5,7,8,9,10,11. The components of a NGGCT can impact the prognosis, with cases of pure choriocarcinoma, yolk sac tumor, or embryonal carcinoma associated with poorer outcomes12. In recent years, immune checkpoint inhibitors have been trialed and approved in the treatment of a variety of malignancies. Immune checkpoint inhibitors are a class of immunotherapy drugs that inhibits co-inhibitory signaling which promotes T-cell mediated anti-cancer immune response. Currently, the most commonly used inhibitors either block the interaction between PD-1 and PD-L1 or via the inhibition of CTLA4. In several adult cancers, the expression of PD-L1 and presence of intratumoral immune infiltrates have been associated with clinical response to immune checkpoint inhibition13.
The expression of PD-L1 and PD-1 in CNS GCT is unclear, especially for NGGCTs. Wildeman et al. found that 90% (19/21 cases) of CNS germinomas had tumor cells that were positive for PD-L114. In contrast, another group reported the absence of PD-L1 in 7 cases of CNS germinomas15. In extracranial GCTs, prior studies have demonstrated conflicting results; some studies reported PD-L1 positivity in tumor cells while others have reported expression in tumor infiltrating lymphocytes and/or macrophages16,17,18.
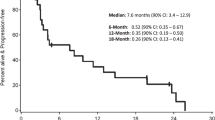
We recently reported a case of a patient with a multiply recurrent NGGCT that had progressed following standard of care treatment who was ultimately treated with checkpoint inhibition19. At his recurrence, the diagnosis of NGGCT (choriocarcinoma) was confirmed histologically, and interestingly, the tumor cells were strongly and diffusely positive for PD-L1 expression on immunohistochemistry (Fig. 1A–C). This patient achieved a durable response to combination therapy with nivolumab and ipilimumab for more than 2 years (Fig. 1D). Given our experience with the index patient with recurrent CNS NGGCT who has had a sustained response to checkpoint blockade, and the paucity of knowledge regarding PD-L1 expression in CNS GCTs, we sought to evaluate PD-L1 and PD-1 expression in these tumors.
Materials and methods
With institutional review board approval, we retrospectively reviewed the pathology archives at our institution for CNS GCTs. A total of 85 patients with 97 neurosurgical resections of CNS GCTs were identified from 1993–2021. Fifty-three of the patients were diagnosed with germinoma, and the remaining 32 patients were diagnosed with a NGGCT. Demographic information, radiologic studies, clinical history, and pathologic report information was extracted from the medical records for each case. In addition, pathology was centrally reviewed by two board-certified neuropathologists (JKW and SA).
Four-μm sections of formalin-fixed, paraffin-embedded tissue were stained with hematoxylin and eosin. Immunohistochemistry (IHC) for lymphocyte markers (CD4, CD8, CD20), PD-1, and PD-L1 was performed on 4-μm sections using the Leica Bond III platform (Leica Biosystems, Buffalo Grove, IL). The following antibodies and methods were used for each protein: CD4 (Clone SP35, Cell Marque, Rocklin, CA, 104R-18, Heat induced epitope retrieval (HIER) 30 min with Bond Epitope Retrieval Solution 2 (ER2)), CD8 (Clone 4B11, Leica Biosystems, PA0183, HIER 20 min with ER2), CD20 (Clone MJ1, Leica Biosystems, PA0906, HIER 20 min with ER2), PD-L1 (Clone SP142, Ventana, Tucson, AZ, 790-4859, HIER 20 min with ER2), and PD-1 (Clone NAT105, Cell Marque, 315M-18, HIER 20 min with ER2). Nuclei were counterstained with hematoxylin, and coverslips were mounted with Permount (Fischer Scientific). Slides were examined with an Olympus BX53 light microscope, and photomicrographs were obtained using an Olympus DP27 camera. PD-L1 was considered positive if membranous staining was present in greater than or equal to 1% of tumor cells, and PD-1 was reported as a percentage of positive inflammatory cells, with greater than 1% counted as positive.
Results
Of the 97 identified cases, 50 had slides or blocks available for histology review and adequate tissue available for IHC (Table 1). For the remainder of the manuscript, only these 50 cases will be discussed. Twenty-eight cases of germinoma were examined from 24 unique patients; 4 cases were recurrences of the primary tumor. For cases of germinoma, the mean age at time of surgery was 15.5 years (range: 10–26 years), and there were 17 males and 11 females. The cases of germinoma were predominantly suprasellar in location (17/28) with the pineal gland being the second most common site (9/28). The remaining two cases were in the thalamus and spinal location, respectively, with the latter being a metastasis of a suprasellar germinoma that was initially biopsied at an outside institution.
Twenty-two cases of NGGCTs were available for review and had tissue available for additional IHC. Of these, there were 17 unique patients, of which 4 had a recurrence and one had two recurrences. For these cases, the mean age at time of surgery was 12.0 years (range: 4–19 years). Twenty-one cases were from male patients, and 1 was a female patient. The NGGCT cases were predominantly pineal (17/22) with 4 cases being suprasellar and 1 case arising in the third ventricle. Of the 22 NGGCT cases, 4 were predominantly teratomas with a germinoma component, and 6 were mostly teratomatous and germinomatous with other minor components, 5 cases were MMGCTs that displayed a mixture of yolk sac tumor, choriocarcinoma, embryonal carcinoma, immature teratoma, and/or germinomatous components, 3 cases were choriocarcinomas, 2 were composed of teratoma and yolk sak tumor, 1 was composed of teratoma and embryonal carcinoma and 1 was an embryonal carcinoma.
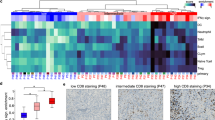
In our cohort, cases of germinoma showed a more prominent lymphocytic infiltrate than NGGCT (Fig. 2). The majority of germinomas showed prominent to moderate amounts of CD4-positive lymphocytes, and a similar to slightly lower quantity of CD8-positive lymphocytes (Fig. 2A). CD20-positive B cells were generally less numerous than T cells in germinomas. The quantity of lymphocytes in NGGCT cases was sparser than that of germinomas. The germinomatous components of the mixed GCTs had moderate to prominent quantities of T cells with fewer B cells as in cases of pure germinoma, but the remainder of NGGCTs only showed rare to scattered quantities of CD4-positive and CD8-positive T cells (Fig. 2B, C). There were even fewer CD20-positive B cells.
A A germinoma with PD-L1 positive in scattered tumor cells and background macrophages, and PD-1 is positive in a subset of lymphocytes but no tumor cells. A typical prominent inflammatory infiltrate is composed of a mixture of CD4- and CD8-positive T cells and fewer CD20-positive B cells. B A choriocarcinoma with prominent PD-L1 staining and scattered PD-1-positive lymphocytes. C A MMGCT with choriocarcinoma components showing positive PD-L1 staining and scattered PD-1-positive lymphocytes. Inflammatory cells were less prominent in NGGCT than in germinomas.
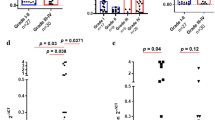
Twenty-two germinomas (79%) and 13 NGGCTs (57%) scored positive for PD-L1 expression in tumor cells. Out of the 22 cases of germinoma that were positive, 13 showed PD-L1 positivity in 1–4% of tumor cells, three cases showed positivity in 5–9%, four cases showed positivity in 10–19%, and two cases showed PD-L1 positivity in 20% or more of tumor cells. In the 13 cases of NGGCT that were positive for PD-L1, the quantity of PD-L1 expression was mixed depending on the type of tumor. The three cases of choriocarcinoma showed strong PD-L1 positivity in at least 50% of tumor cells (Fig. 3). Mixed GCT cases showed positivity in the germinoma and choriocarcinoma components as described in the pure tumors. In the cases of mixed GCTs, the teratomatous as well as embryonal carcinoma components were negative for PD-L1. In addition to the PD-L1 positivity seen in germinoma and choriocarcinoma, yolk sac tumors also displayed PD-L1 positivity in rare cells. PD-1 expression was seen in at least 1% of lymphocytes in 27/28 of the germinomas. Of the 27 cases of germinoma with positive PD-1 expression in lymphocytes, the majority of these cases showed PD-1 expression between 5–20% of the total lymphocyte population. PD-1 expression was seen in lymphocytes of 20/23 of the NGGCTs, with the majority of cases showing between 5–10% of lymphocytes expressing PD-1. Of note, we did not observe PD-1 positivity in tumor cells of either germinomas or NGGCTs (Table 2).
Out of the 50 cases with available tissue, there were samples from nine recurrent surgeries; four of these were germinomas and five were NGGCTs. In the four cases of recurrent germinoma, three showed increased expression of PD-L1 on the second surgery, and one case showed decreased PD-L1 expression. For recurrent NGGCTs, the expression of PD-L1 decreased or stayed the same in subsequent surgeries. One patient’s tumor remained negative, and two patients had low level PD-L1 expression in tumor cells in the first surgery and were subsequently negative on the recurrence. A single patient had three surgeries, and his tumor showed decreasing levels of PD-L1 expression in each surgery.
Discussion
In this paper we described the expression of checkpoint markers, specifically PD-L1 and PD-1 in GCTs of the CNS. Most cases of CNS germinoma had positive PD-L1 expression in tumor cells, consistent with a previous report14. Cases of choriocarcinoma showed strong and diffuse expression of PD-L1, as had been reported in extracranial GCTs16. In addition, we reported PD-1-positive lymphocytes present in the majority of germinomas and NGGCTs, with larger numbers present in germinomas.
Although numerous studies have evaluated PD-L1 expression in both extracranial and CNS GCTs, knowledge about response to checkpoint inhibitors remains limited. Our index patient had a pineal choriocarcinoma and showed a durable response to ipilimumab and nivolumab19. He was later placed on nivolumab monotherapy, and his tumor remained stable on imaging for over 2 years after starting therapy (Fig. 1D). Zschabitz et al. reported that two of seven patients with extracranial GCTs treated with checkpoint inhibitors (nivolumab or pembrolizumab) had a durable response20. Both of these patients had tumors with strong PD-L1 staining. Another case report of a patient with widely metastatic choriocarcinoma treated with nivolumab showed a durable response and stable, low levels of β-hCG21. However, not all patients with choriocarcinoma respond to checkpoint inhibitors, as a case report of a different patient with metastatic choriocarcinoma treated with pembrolizumab showed rapid progression after one cycle22. In testicular GCTs, higher expression of PD-L1 has been suggested to correlate with worse prognostic features23, and the same group reported a better prognosis in cases of testicular GCTs with higher numbers of PD-L1-positive tumor and PD1-positive infiltrating lymphocytes17.
Although our cohort is from a single institution and the number of cases of each type of tumor is relatively small, this study is the largest cohort of CNS GCTs analyzed for PD-L1 expression, and the first one to characterize the PD-L1 expression in the different GCT components in the brain. Our results and the index case previously reported by our institution suggest that checkpoint inhibition could be a possible treatment for CNS GCTs. Currently, immune checkpoint inhibitors do not surpass conventional therapy in pediatric patients, but they are utilized in situations in which conventional or gene targeted therapies have provided limited benefit. Based on IHC for PD-L1 and high rates of positivity, cases of choriocarcinoma and germinoma would likely benefit the most from treatment with checkpoint blockade. In our pathology practice, IHC for PD-L1 and PD-1 is not performed routinely; it is usually performed at the request of the treating clinical team. In addition to PD-L1 expression, other biomarkers including tumor mutational burden (TMB) have been shown to correlate with increased benefit from checkpoint inhibitors13. The mechanism has been proposed to relate to neoantigen presentation, and assessment of this in patients would be difficult to implement in clinical practice. Interestingly our index case did not have a high mutational burden (3.0 mutations per megabase), suggesting that a high TMB may not be necessary for response in CNS choriocarcinomas or other GCTs.
Despite not being in the first-line panel for evaluating CNS GCTs, PD-1 and PD-L1 immunostains remain an inexpensive method of investigating suitability for a particular therapy. However, the therapy’s efficacy and toxicity in pediatric patients still needs exploration in clinical trials. Previous clinical trials have reported little efficacy in treating refractory extracranial GCTs24. In one clinical trial, no partial or complete response was achieved in 12 patients, and only 2 patients showed stable disease on imaging but had increasing levels of alpha-fetoprotein25. In another phase II clinical trial, eight patients with refractory, extracranial GCTs all showed progressive disease after 12 weeks of treatment with avelumab26. Currently there is an ongoing clinical trial to assess checkpoint inhibitors in recurrent, rare CNS tumors including GCTs (NCT03173950), but a multi-center trial focused solely on CNS GCTs will be needed to determine the appropriate clinical setting for using checkpoint inhibitors in patients with intracranial disease.
It is worth noting that some patients in our cohort received treatment prior to their first surgery, which may affect PD-L1 expression in some types of cancer27. Also, there is some reported variability in immunohistochemical results when utilizing different clones of PD-L1 antibody22,28; our institution uses the SP142 antibody which has been FDA approved as a companion diagnostic method for treatment of urothelial carcinoma with Atezolizumab29.
In summary, we describe the PD-L1 and PD1 expression in CNS GCT and highlight their patterns of expression in choriocarcinoma and embryonal carcinoma, which may be of importance when therapy with immune checkpoint inhibitors is a consideration.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
McCarthy, B. J., Shibui, S., Kayama, T., Miyaoka, E., Narita, Y., Murakami, M. et al. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro Oncol 14, 1194-1200 (2012).
Rosenblum MK, Ichimura K, Pietsch T, Lau CC, Nishikawa R, Wong TT. Germ cell tumors of the CNS. WHO Classification of Tumours Editorial Board. Central Nervous System Tumours. 5th edition, chapter 11 (2021).
Ostrom, Q. T., Francis, S. S. & Barnholtz-Sloan, J. S. Epidemiology of Brain and Other CNS Tumors. Curr Neurol Neurosci Rep 21, 68 (2021).
Fetcko, K. & Dey, M. Primary Central Nervous System Germ Cell Tumors: A Review and Update. Med Res Arch 6 (2018).
Calaminus, G., Kortmann, R., Worch, J., Nicholson, J. C., Alapetite, C., Garre, M. L. et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 15, 788-796 (2013).
Lo, A. C., Hodgson, D., Dang, J., Tyldesley, S., Bouffet, E., Bartels, U. et al. Intracranial Germ Cell Tumors in Adolescents and Young Adults: A 40-Year Multi-Institutional Review of Outcomes. Int J Radiat Oncol Biol Phys 106, 269-278 (2020).
Fangusaro, J., Wu, S., MacDonald, S., Murphy, E., Shaw, D., Bartels, U. et al. Phase II Trial of Response-Based Radiation Therapy for Patients With Localized CNS Nongerminomatous Germ Cell Tumors: A Children’s Oncology Group Study. J Clin Oncol 37, 3283-3290 (2019).
Calaminus, G., Frappaz, D., Kortmann, R. D., Krefeld, B., Saran, F., Pietsch, T. et al. Outcome of patients with intracranial non-germinomatous germ cell tumors-lessons from the SIOP-CNS-GCT-96 trial. Neuro Oncol 19, 1661-1672 (2017).
Goldman, S., Bouffet, E., Fisher, P. G., Allen, J. C., Robertson, P. L., Chuba, P. J. et al. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: a Children’s Oncology Group study. J Clin Oncol 33, 2464-2471 (2015).
Echevarria, M. E., Fangusaro, J. & Goldman, S. Pediatric central nervous system germ cell tumors: a review. Oncologist 13, 690-699 (2008).
Murray, M. J., Bartels, U., Nishikawa, R., Fangusaro, J., Matsutani, M., Nicholson, J. C. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol 16, e470-e477 (2015).
Dufour, C., Guerrini-Rousseau, L. & Grill, J. Central nervous system germ cell tumors: an update. Curr Opin Oncol 26, 622-626 (2014).
Buder-Bakhaya, K. & Hassel, J. C. Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment-A Review From the Melanoma Perspective and Beyond. Front Immunol 9, 1474 (2018).
Wildeman, M. E., Shepard, M. J., Oldfield, E. H. & Lopes, M. B. S. Central Nervous System Germinomas Express Programmed Death Ligand 1. J Neuropathol Exp Neurol 77, 312-316 (2018).
Aoki, T., Hino, M., Koh, K., Kyushiki, M., Kishimoto, H., Arakawa, Y. et al. Low Frequency of Programmed Death Ligand 1 Expression in Pediatric Cancers. Pediatr Blood Cancer 63, 1461-1464 (2016).
Fankhauser, C. D., Curioni-Fontecedro, A., Allmann, V., Beyer, J., Tischler, V., Sulser, T. et al. Frequent PD-L1 expression in testicular germ cell tumors. Br J Cancer 113, 411-413 (2015).
Chovanec, M., Cierna, Z., Miskovska, V., Machalekova, K., Svetlovska, D., Kalavska, K. et al. Prognostic role of programmed-death ligand 1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular germ cell tumors. Oncotarget 8, 21794-21805 (2017).
Sadigh, S., Farahani, S. J., Shah, A., Vaughn, D. & Lal, P. Differences in PD-L1-Expressing Macrophages and Immune Microenvironment in Testicular Germ Cell Tumors. Am J Clin Pathol 153, 387-395 (2020).
Cacciotti, C., Choi, J., Alexandrescu, S., Zimmerman, M. A., Cooney, T. M., Chordas, C. et al. Immune checkpoint inhibition for pediatric patients with recurrent/refractory CNS tumors: a single institution experience. J Neurooncol 149, 113-122 (2020).
Zschabitz, S., Lasitschka, F., Hadaschik, B., Hofheinz, R. D., Jentsch-Ullrich, K., Gruner, M. et al. Response to anti-programmed cell death protein-1 antibodies in men treated for platinum refractory germ cell cancer relapsed after high-dose chemotherapy and stem cell transplantation. Eur J Cancer 76, 1-7 (2017).
Chi, E. A. & Schweizer, M. T. Durable Response to Immune Checkpoint Blockade in a Platinum-Refractory Patient With Nonseminomatous Germ Cell Tumor. Clin Genitourin Cancer 15, e855-e857 (2017).
Loh, K. P. & Fung, C. Novel Therapies in Platinum-refractory Metastatic Germ Cell Tumor: A Case Report with a Focus on a PD-1 Inhibitor. Rare Tumors 9, 6867 (2017).
Cierna, Z., Mego, M., Miskovska, V., Machalekova, K., Chovanec, M., Svetlovska, D. et al. Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann Oncol 27, 300-305 (2016).
Kalavska, K., Schmidtova, S., Chovanec, M. & Mego, M. Immunotherapy in Testicular Germ Cell Tumors. Front Oncol 10, 573977 (2020).
Adra, N., Einhorn, L. H., Althouse, S. K., Ammakkanavar, N. R., Musapatika, D., Albany, C. et al. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: a Hoosier Cancer Research Network Study GU14-206. Ann Oncol 29, 209-214 (2018).
Mego, M., Svetlovska, D., Chovanec, M., Reckova, M., Rejlekova, K., Obertova, J. et al. Phase II study of avelumab in multiple relapsed/refractory germ cell cancer. Invest N Drugs 37, 748–754 (2019).
Fujimoto, D., Uehara, K., Sato, Y., Sakanoue, I., Ito, M., Teraoka, S. et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci Rep 7, 11373 (2017).
Xu, H., Lin, G., Huang, C., Zhu, W., Miao, Q., Fan, X. et al. Assessment of Concordance between 22C3 and SP142 Immunohistochemistry Assays regarding PD-L1 Expression in Non-Small Cell Lung Cancer. Sci Rep 7, 16956 (2017).
Eckstein, M., Cimadamore, A., Hartmann, A., Lopez-Beltran, A., Cheng, L., Scarpelli, M. et al. PD-L1 assessment in urothelial carcinoma: a practical approach. Ann Transl Med 7, 690 (2019).
Acknowledgements
We would like to thank the Histology Laboratory at Boston Children’s Hospital for their technical assistance. The authors would like to thank Susan I. Permut for her support of this research study.
Funding
JKW currently receives salary support from an NIH Institutional Training Grant (T32 CA251062), however there was no direct NIH funding utilized for this study.
Author information
Authors and Affiliations
Contributions
JKW gathered the cohort, analyzed the clinical and pathology data, prepared the figures and tables, and wrote the manuscript. HGL, KLL, SS, and SNC participated in the care of many of the patients in the cohort and edited the manuscript. KKY participated in the study design and cohort selection and contributed to the write-up of the manuscript. SA mentored JKW through all the phases of the project, reviewed the pathology and the clinical data, edited the manuscript, and prepared the project for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woods, J.K., Lidov, H.G., Ligon, K.L. et al. PD-L1 and PD-1 expression in pediatric central nervous system germ cell tumors. Mod Pathol 35, 1770–1774 (2022). https://doi.org/10.1038/s41379-022-01142-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01142-3