Abstract
Invasive lobular breast cancer (ILC) is a special breast cancer (BC) subtype and is mostly hormone receptor (HR)-positive and ERBB2 non-amplified. Endocrine therapy restrains tumor proliferation and is the mainstay of lobular BC treatment. Mutation of ERBB2 has been associated with recurrent ILC. However, it is unknown whether ERBB2 mutation impacts on the otherwise exquisite responsiveness of early ILC to endocrine therapy. We have recently profiled n = 622 HR-positive early BCs from the ADAPT trial for mutations in candidate genes involved in endocrine resistance, including ERBB2. All patients were treated with short-term preoperative endocrine therapy (pET, tamoxifen or aromatase inhibitors) before tumor resection. Tumor proliferation after endocrine therapy (post-pET Ki67 index) was determined prospectively by standardized central pathology assessment supported by computer-assisted image analysis. Sustained or suppressed proliferation were defined as post-pET Ki67 ≥10% or <10%. Here, we report a subgroup analysis pertaining to ILCs in this cohort. ILCs accounted for 179/622 (28.8%) cases. ILCs were enriched in mutations in CDH1 (124/179, 69.3%, P < 0.0001) and ERBB2 (14/179, 7.8%, P < 0.0001), but showed fewer mutations in TP53 (7/179, 3.9%, P = 0.0048) and GATA3 (11/179, 6.1%, P < 0.0001). Considering all BCs irrespective of subtypes, ERBB2 mutation was not associated with proliferation. In ILCs, however, ERBB2 mutations were 3.5-fold more common in cases with sustained post-pET proliferation compared to cases with suppressed post-pET proliferation (10/75, 13.3% versus 4/104, 3.8%, P = 0.0248). Moreover, ERBB2 mutation was associated with high Oncotype DX recurrence scores (P = 0.0087). In summary, our findings support that ERBB2 mutation influences endocrine responsiveness in early lobular BC.
Similar content being viewed by others
Introduction
Invasive lobular breast cancer (ILC) is a special histological BC subtype1,2. Lobular BC is typically hormone receptor (HR)-positive and HER2/ERBB2 non-amplified1. Lobular BC is driven by mutational inactivation of CDH1/E-cadherin and is a slow-growing tumor entity3,4. Endocrine therapy (ET) restrains ILC cell proliferation and is the mainstay of systemic treatment for lobular BC in the clinic5,6. Activating mutation of ERBB2 has been associated with recurrent and metastatic ILC and poor prognosis7,8,9,10,11,12,13,14. Estrogen receptor (ER)-positive human BC cell lines with isogenically incorporated ERBB2 mutation acquire resistance to growth inhibition by estrogen deprivation in vitro15. Accordingly, it is thought that ERBB2 mutation mediates endocrine resistance in vitro10,15.
However, it is currently unknown whether or how much ERBB2 mutation impacts on the otherwise exquisite clinical responsiveness of ILC cells to endocrine therapy, especially in early ILC. Assessment of the ERBB2 mutation status is not a standard diagnostic procedure in the clinic16. Furthermore, assessment of tumor cell proliferation in early BC pre-treated with endocrine therapy requires that anti-hormonal therapy is initiated a short time before tumor resection. This is not usually done in the clinic. Short-term preoperative endocrine therapy (pET) does not improve outcome, but it offers an empirical readout for endocrine responsiveness by measuring the post-pET Ki67 cell proliferation index17,18,19. So far, pET has only been implemented in a limited number of prospective clinical trials, including the POETIC and ADAPT trial17,18,19,20,21,22. Whether or not ERBB2 mutation impacts on tumor cell proliferation in early ILC treated with preoperative endocrine therapy has not yet been investigated in this context. Therefore, we extended our previous, retrospective exploratory analyses of BCs from the ADAPT trial (HR-positive/HER2-negative)23. Here we report on the relation between genetic alterations and tumor cell proliferation following pET, with a special reference to ILC and ERBB2 mutation.
Materials and methods
Tumor specimens
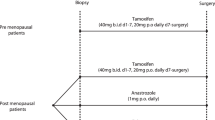
Tumor tissues included n = 622 HR-positive/HER2-negative early BCs from patients enrolled in the West German Study Group (WSG) ADAPT trial (NCT01779206). This cohort corresponds to a subset of patients enrolled in the ADAPT trial (run-in phase)21,22,23. Study design details were reported previously21,22,24. Short-term preoperative endocrine therapy (pET; with tamoxifen [TAM] or aromatase inhibitors [AIs]) was administered for three weeks before tumor resection21,22. Oncotype DX recurrence score (RS) testing at baseline was performed at the laboratory of Genomic Health Inc (Redwood City, CA, USA). All tumors were subjected to prospective central pathology review (MHH, Hannover, Germany) for histological subtyping and prospective assessment of tumor cell proliferation (documented in 2012–2016). Histologic BC subtypes were determined in accordance with the criteria of the “World Health Organization (WHO) classification of tumours of the breast”, 4th edition (2012)25. Histologic BC subtype calls were based on a consensus of three experts headed by H.K. and were aided by upfront E-cadherin immunohistochemistry (IHC), as described recently26. A comprehensive description of criteria for the diagnosis of ILC has been provided elsewhere1,2. From an initial set of n = 701 case, n = 79 cases were excluded from further molecular work-up (performed in 2018–2020) due to (i) missing Ki67 values, (ii) unavailable tissue blocks (returned to local centers upon clinical request), (iii) divergent histological subtypes at baseline or post-pET or controversial classification as either lobular or non-lobular BC, including cases classifiable as BC with mixed ductal/lobular features (n = 12), (iv) triple-negative hormone receptor status, (v) insufficient DNA amount and/or quality23. The total number of cases for the final statistical analysis was n = 622 (Table 1). The characteristics of the study population included in this retrospective molecular analysis were reported earlier23. This study was approved by the local ethic committee (MHH, Hannover, ID 2716–2015).
Immunohistochemistry and assessment of tumor cell proliferation
Immunohistochemistry (IHC) for estrogen receptor (ER), progesterone receptor (PR) and HER2 was performed prospectively in the central pathology unit of the ADAPT trial as described previously23. BCs scored as HER2 2+ and 3+ were subjected to HER2/ERBB2 fluorescence in situ hybridization in accordance with ASCO/CAP guidelines16. Tumor cell proliferation before endocrine therapy (baseline) and after endocrine therapy (post-pET) was determined prospectively by standardized central pathology assessment of the Ki67 cell proliferation marker18,19,23. Immunohistochemical staining of Ki67 was performed with the anti-Ki67 antibody clone 30-9 (Ventana, Tucson, AZ, USA)23,27,28. Ki67 scoring was supported by computer-assisted image analysis (iScan Coreo slide scanner and Virtuoso v5.3 software for digital quantification, Ventana, Tucson, AZ, USA)29,30. The Ki67 index was based on three independent evaluations (2x semiquantitative assessments by experienced pathologists, 1x digital quantification with Virtuoso software)22,23. The semiquantitative Ki67 index that was nearest to the digital Ki67 index was accepted as the consensus Ki67 index22,23. Representative immunohistochemical stainings of BCs in each Ki67 category (0–9%, 10–19%, 20–34%, and 35–100%) are shown in the data supplement (Supplementary Fig. 1A). The MKI67 gene is one of the key determinants of the Oncotype DX recurrence scores31. Higher baseline Ki67 indices were well correlated with higher recurrence scores, which indirectly substantiated the validity of Ki67 assessment by IHC (Supplementary Fig. 1B)17. Sustained and suppressed tumor cell proliferation after therapy were defined as post-pET Ki67 ≥10% and <10%23. This cutoff represents a provisional cutoff that was implemented only for retrospective molecular analyses in the ADAPT translation research program23. This cutoff is consistent with similar analyses in the POETIC trial18. In the POETIC trial, post-pET Ki67 indices of ≥10% and <10% were termed high and low post-treatment proliferation, respectively18. E-cadherin protein expression was determined with the anti-E-cadherin antibody ECH-6 (Zytomed Systems, Berlin, Germany)26. E-cadherin IHC was scored as negative (complete loss) versus positive (any specific staining). All IHC stainings were performed on a Benchmark Ultra (Ventana, Tucson, AZ, USA) automated stainer.
DNA extraction and mutational analysis
Extraction of DNA and analysis of genetic alterations were performed (in 2018-2020) as described previously23. In addition, mutational analysis of the CDH1 gene was carried out by next generation sequencing (NGS) using a customized NGS panel, which covered the complete protein-coding sequence including 10 bp of the flanking intron sequence of the CDH1 gene. Mean mapped reads per sample was 282,620 (range 33,486 to 7,149,110). Data processing and evaluation were performed as described previously23.
Statistics
For statistical evaluation of the association between genetic alterations and pathologic parameters, we focused on candidate genes with a mutation frequency of ≥2.5%. The two-sided Fisher’s exact test and the Chi square test for trends were used for contingency analyses. The Mann–Whitney test was used to determine statistical significance of different median Ki67 indices in BC subsets. Results were considered as statistically significant if P ≤ 0.0500. Statistical analyses were performed with GraphPad Prism software Version 5.00 (GraphPad Software, San Diego, CA, USA).
Results
Baseline characteristics
We have recently profiled n = 622 HR-positive early BCs from the ADAPT trial for genetic alterations in selected candidate genes involved in endocrine resistance or responsiveness23. All patients were treated with short-term pET (TAM or AI) for three weeks before tumor resection21,22. Here, we report a subgroup analysis pertaining to the lobular BCs included in this cohort. According to central pathology review, lobular BC accounted for 179/622 (28.8%) cases. ILC was associated with larger pT stage, lower histological grade, lower Oncotype DX recurrence scores, lower baseline Ki67 (before pET), and loss of E-cadherin (Table 1). This is consistent with previous studies32.
Genetic alterations in ILC
Genes previously assessed in this cohort included ARID1A, BRAF, ERBB2, ESR1, GATA3, HRAS, KRAS, NRAS, PIK3CA, and TP53 (mutational analysis by NGS), and CCND1, FGFR1 and PAK1 (copy number assessment by digital PCR)23. These genes were selected as candidate genes involved in endocrine tumor response based on the study of Razavi et al., which focused on metachronous BC recurrences after failure of (adjuvant) ET10,23. For the present subgroup analysis of ILCs we also performed mutational analyses of CDH1. As expected, ILCs were enriched in cases harboring mutations in CDH1 (124/179, 69.3%, P < 0.0001) (Fig. 1A, B). In contrast, CDH1 mutations were rare in non-lobular BC (11/443, 2.5%) (Fig. 1A, B). Furthermore ILCs were enriched in mutations in ERBB2 (14/179, 7.8%, P < 0.0001), but showed fewer mutations in TP53 (7/179, 3.9%, P = 0.0048) and GATA3 (11/179, 6.1%, P < 0.0001) (Fig. 1C, D, Supplementary Table 1). Regarding mutation types, CDH1 mutations were mostly nonsense and frameshift mutations (114/135, 84.4%) (Fig. 1B). CDH1 mutations were nearly always accompanied by loss of E-cadherin (115/128, 89.8%) (Fig. 2A). CDH1 missense mutations associated with preserved E-cadherin expression were rare (7/128, 5.5%) (Supplementary Fig. 2). Most ERBB2 mutations were missense mutations (15/18, 83.3%) resulting in single amino acid substitutions in the tyrosine kinase domain, such as p.L755S (Fig. 1B and Supplementary Table 2). The remaining ERBB2 mutations (3/18, 16.7%) were exon 20 in-frame insertions or duplications, such as p.Y772_A775dup, which are more common in non-small cell lung cancer (NSCLC) and induce a constitutively active ERBB2 protein conformation (Supplementary Table 2)33. One ILC (case 110290) harbored two different ERBB2 mutations (p.I767M and p.V777L) (Supplementary Table 2). In total, 12/18 (66.7%) ERBB2-mutated BCs harbored a concomitant CDH1 mutation (P < 0.0001), confirming the strong association of these two alterations, as reported earlier (Fig. 2B)8. Overall, mutation frequencies were consistent with independent previous studies3,34,35,36. However, our BC cohort showed slightly more CDH1 and ERBB2 mutations in ILC, and more GATA3 mutations in non-lobular BC than reported in previous studies (Supplementary Fig. 3 and Supplementary Table 3).
Bar charts showing the frequencies of genetic alterations in candidate genes involved in endocrine tumor response in non-lobular (A) and lobular BC (B). A scatter plot illustrating the frequencies gene mutations in non-lobular (x-axis) versus lobular BC (y-axis) is shown in the lower panel (C). A magnified view of the inset is provided on the right side (D). The color of the dots reflect statistical significance (of frequencies in non-lobular BC versus lobular BC). Colored dots correspond to statistically significant differences with P values of <0.0500, and <0.0010, respectively. ins insertion, del deletion.
A Venn diagram showing the overlap between lobular BC, CDH1 mutation, and loss of E-cadherin, as determined by IHC with the anti-E-cadherin antibody ECH-6. Please note that the E-cadherin IHC status was not available for 35/622 patients. B Venn diagram showing the overlap between lobular BC, CDH1 mutation, and ERBB2 mutation. Numbers within the Venn diagram indicate numbers of patients/cases.
Relation between post-pET Ki67 and ERBB2 mutation in ILC
An advantage of the BC collection described herein is that patients were treated with short-term pET before surgery21. Furthermore, post-pET tumor cell proliferation was prospectively determined by standardized central assessment of the Ki67 index in the framework of a controlled clinical trial (ADAPT)21. Moreover, central Ki67 scoring was supported by computer-assisted image analysis23. No such comprehensive information on post-pET proliferation is available for specimens in public BC mutation databases, such as METABRIC or TCGA4. Representative immunohistochemical stainings for Ki67 at baseline and post-pET are shown in Fig. 3 (Fig. 3). Our previous analyses in this BC cohort (n = 622) revealed that sustained tumor cell proliferation after preoperative endocrine therapy (defined as post-pET Ki67 ≥10%) was associated with TP53 mutation but not with ERBB2 mutation23. In fact, by considering all BCs irrespective of subtypes, ERBB2 mutation was not associated with any prognostic parameter or enhanced proliferation (Supplementary Table 4). This was surprising, given that mutant ERBB2 mediates estrogen-independent proliferation in ER-positive BC cell lines in vitro15. ERBB2 mutation was also not associated with lower ER or PR expression or higher histological grade at baseline (before pET) (Supplementary Figs. 4 and 5, and Supplementary Table 4).
Shown are three representative lobular BCs (IDs 220244, 220048, and 220473). Case IDs, CDH1 and ERBB2 mutations and Oncotype DX recurrence scores are indicated at the left margin. The left panels show HE- and Ki67-stained core needle biopsies (CNBs) before treatment (baseline) at x200 magnification (scale bar corresponds to 200 µm). The right panels show HE- and Ki67-stained resection specimens of the same tumors after per-operative endocrine therapy (post-pET). Insets in the upper right corners indicate the consensus Ki67 index (con. Ki67 [% positive tumor cells]) obtained by semiquantitative assessment by two experienced pathologist and digital quantification using computer-assisted image analysis (Virtuoso v5.3 software), as described in the materials and methods section. con consensus, RS recurrence score, wt wild-type.
As ERBB2 mutation occurred mostly in ILC, and because lobular BCs are mostly slow growing tumors, we hypothesized that the impact of ERBB2 mutation on post-pET proliferation might not be apparent, if ILC and non-lobular BCs are lumped together for statistical analysis. Accordingly, we conducted exploratory subgroup analyses to specify genetic alterations associated with post-pET Ki67 in lobular and non-lobular BC respectively (Supplementary Table 5). Strikingly, sustained tumor cell proliferation after preoperative endocrine therapy (post-pET Ki67 ≥10%) was associated with TP53 mutation in non-lobular BC (Fig. 4A) but with ERBB2 mutation in ILC (Fig. 4B). In detail, ERBB2 mutations were 3.5-fold more common in ILCs with sustained post-pET proliferation compared to ILCs with suppressed post-pET proliferation (10/75, 13.3% versus 4/104, 3.8%, P = 0.0248) (Fig. 4B). Conversely, failure of pET to achieve optimal suppression of tumor cell proliferation (post-pET Ki67 < 10%) was 1.8-fold more common in ERBB2-mutated ILC compared to ILC harboring wild-type ERBB2 (10/14, 71% versus 65/165, 39%, P = 0.0248). This association was also statistically significant in the subset of ILC patients that were treated with AI for pET (Supplementary Table 5). Furthermore, ERBB2 mutations in ILCs were associated with the “high-high” dynamic Ki67 category (baseline and post-pET Ki67 both ≥10%), as defined recently in the POETIC trial (P = 0.0263) (Supplementary Fig. 6 and Supplementary Table 6)18. ILCs harboring ERBB2 mutations also showed a borderline significant trend towards a higher median post-pET Ki67 index (P = 0.0513) (Supplementary Fig. 7). Particularly high baseline and post-pET Ki67 indices (both 30%) were observed in one ILC that harbored two different ERBB2 mutations (case 110290) (Supplementary Table 2). Moreover, ERBB2 mutation was associated with high risk Oncotype DX recurrence scores (P = 0.0087), but only in the subgroup of lobular BCs (Fig. 4C).
A Bar chart showing the TP53 mutation frequency among cases with post-pET Ki67 < 10% (gray) or ≥10% (dark gray). B Bar chart showing the ERBB2 mutation frequency among cases with post-pET Ki67 < 10% (gray) or ≥10% (dark gray). C Bar chart showing the ERBB2 mutation frequency among cases with low/intermediate Oncotype DX recurrence score (RS 0–11 plus RS 12–25, light gray), or high recurrence score (RS 26–100, dark gray). Statistical significance was determined with Fisher’s Exact Test. RS recurrence score, pET preoperative endocrine therapy.
Concordant detection of ERBB2 mutation in matched baseline and post-pET specimens
ERBB2 mutations may arise de novo during endocrine therapy10. In our BC cohort, ERBB2 mutations were concordantly detected in resections specimens (post-pET) and matched core needle biopsies (baseline, before pET) in 14/18 (77.8%) cases tested (Supplementary Table 2). This indicates that most ERBB2 mutations were already present at baseline, in treatment-naïve BCs.
Discussion
Lobular BC is a distinct tumor entity characterized by a special histomorphology, distinct genetic alterations, including CDH1 mutation, and comparatively slow, estrogen-dependent growth1,3,4. Activating mutation of ERBB2 has been associated with recurrent and metastatic ILC and poor prognosis7,8,9,10,11,12. Based on in vitro models, it is thought that mutant ERBB2 mediates hormone-independent cell proliferation and thus resistance to endocrine therapy15. This appears to explain the overrepresentation of ERBB2-mutated ILC among patients with tumor recurrences after adjuvant endocrine therapy7,10. However, up to now, there was only limited or no evidence that ERBB2 mutation actually does make a change for tumor cell proliferation in patients receiving endocrine therapy, especially in early lobular BC. The limited availability of tumor specimens from patients that have been treated with short-term pET before surgery for early BC may be one of the reasons for the lack of studies in this direction17,18,19.
In the present study we have extended our previous molecular analyses of BC specimens from the ADAPT (HR-positive/HER2-negative) trial, in which all patients were treated with pET before surgery21,22,23. ILCs included in this cohort were strongly enriched in CDH1 mutations and ERBB2 mutations. This is in line with previous studies3,8,34,35,36. Considering all BCs irrespective of subtypes, ERBB2 mutation did not show any significant association with prognostic parameters or tumor cell proliferation. In the subgroup of ILCs, however, ERBB2 mutations were enriched 3.5-fold in cases with sustained post-pET proliferation compared to cases with suppressed post-pET proliferation (13.3% versus 3.8%, P = 0.0248). Moreover, ERBB2 mutation was associated with high risk Oncotype DX recurrences scores, but only in the subgroup of lobular BC.
Limitations of the present study include: (i) the retrospective approach of molecular analyses, (ii) the exploratory subgroup analysis for BCs with lobular histology, (iii) exclusion of a small minority of cases with controversial subtype calls (including cases classifiably as BC with mixed ductal/lobular features) and (iv) the comparatively small absolute number of ERBB2-mutated cases. However, compared with the situation in early HR-positive/HER2-positive BCs, there is a lack of prospective clinical trials designed specifically for early HR-positive and/or ERBB2-mutant ILC. Accordingly, retrospective exploratory subgroup analyses may represent a necessary intermediate step on the way towards new clinical trials for this distinct tumor type.
In fact, the findings reported here are important for two reasons. First, this study further supports that mutant ERBB2 influences endocrine responsiveness in lobular BC. For the first time, this has now also been demonstrated by means of clinical tumor specimens obtained after pET. Even so, a proportion of ERBB2-mutant ILCs still showed optimal suppression of tumor cell proliferation by pET. Accordingly, it remains an open question whether ERBB2 mutation is a suitable prognostic factor that is independent from other parameters, such as the Onctotype DX recurrence score. Further analyses in larger cohorts treated with adjuvant endocrine therapy alone, or with adjuvant endocrine therapy plus adjuvant chemotherapy would be necessary to clarify this issue. The current retrospective, exploratory subgroup analysis of a proportion of patients enrolled in the ADAPT trial (run-in phase) was not sufficiently powered to address this problem. Second, this subgroup analysis exemplifies that biologically relevant associations may be masked and can remain unidentified, if HR-positive ILCs and non-lobular BCs are lumped together for statistical analyses in BC research. Accordingly, lobular BC deserves greater attention in clinical trials and translational research.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Christgen M, Cserni G, Floris G, Marchio C, Djerroudi L, Kreipe H, et al. Lobular Breast Cancer: Histomorphology and Different Concepts of a Special Spectrum of Tumors. Cancers (Basel) 13, (2021)
Christgen M, Steinemann D, Kuhnle E, Langer F, Gluz O, Harbeck N, et al. Lobular breast cancer: Clinical, molecular and morphological characteristics. Pathol Res Pract 212, 583-97 (2016)
Desmedt C, Zoppoli G, Gundem G, Pruneri G, Larsimont D, Fornili M, et al. Genomic Characterization of Primary Invasive Lobular Breast Cancer. J Clin Oncol 34, 1872-81 (2016)
McCart Reed AE, Kalinowski L, Simpson PT, Lakhani SR Invasive lobular carcinoma of the breast: the increasing importance of this special subtype. Breast Cancer Res 23, 6 (2021)
Riggins RB, Lan JP, Klimach U, Zwart A, Cavalli LR, Haddad BR, et al. ERRgamma mediates tamoxifen resistance in novel models of invasive lobular breast cancer. Cancer Res 68, 8908-17 (2008)
Conforti F, Pala L, Pagan E, Viale G, Bagnardi V, Peruzzotti G, et al. Endocrine-responsive lobular carcinoma of the breast: features associated with risk of late distant recurrence. Breast Cancer Res 21, 153 (2019)
Ross JS, Wang K, Sheehan CE, Boguniewicz AB, Otto G, Downing SR, et al. Relapsed classic E-cadherin (CDH1)-mutated invasive lobular breast cancer shows a high frequency of HER2 (ERBB2) gene mutations. Clin Cancer Res 19, 2668-76 (2013)
Ross JS, Gay LM, Wang K, Ali SM, Chumsri S, Elvin JA, et al. Nonamplification ERBB2 genomic alterations in 5605 cases of recurrent and metastatic breast cancer: An emerging opportunity for anti-HER2 targeted therapies. Cancer 122, 2654-62 (2016)
Christgen M, Bartels S, Luft A, Persing S, Henkel D, Lehmann U, et al. Activating human epidermal growth factor receptor 2 (HER2) gene mutation in bone metastases from breast cancer. Virchows Arch 473, 577-82 (2018)
Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 34, 427-38 e6 (2018)
Kurozumi S, Alsaleem M, Monteiro CJ, Bhardwaj K, Joosten SEP, Fujii T, et al. Targetable ERBB2 mutation status is an independent marker of adverse prognosis in estrogen receptor positive, ERBB2 non-amplified primary lobular breast carcinoma: a retrospective in silico analysis of public datasets. Breast Cancer Res 22, 85 (2020)
McCart Reed AE, Foong S, Kutasovic JR, Nones K, Waddell N, Lakhani SR, et al. The genomic landscape of lobular breast cancer. Cancers (Basel) 13, 1950 (2021)
Aftimos P, Oliveira M, Irrthum A, Fumagalli D, Sotiriou C, Gal-Yam EN, et al. Genomic and Transcriptomic Analyses of Breast Cancer Primaries and Matched Metastases in AURORA, the Breast International Group (BIG) Molecular Screening Initiative. Cancer Discov 11, 2796-811 (2021)
Bose R, Ma CX Breast Cancer, HER2 Mutations, and Overcoming Drug Resistance. N Engl J Med 385, 1241-43 (2021)
Croessmann S, Formisano L, Kinch LN, Gonzalez-Ericsson PI, Sudhan DR, Nagy RJ, et al. Combined Blockade of Activating ERBB2 Mutations and ER Results in Synthetic Lethality of ER+/HER2 Mutant Breast Cancer. Clin Cancer Res 25, 277-89 (2019)
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 36, 2105-22 (2018)
Dowsett M Testing endocrine response for managing primary estrogen receptor-positive breast cancer. J Clin Oncol In press, (2022)
Smith I, Robertson J, Kilburn L, Wilcox M, Evans A, Holcombe C, et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol 21, 1443-54 (2020)
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99, 167-70 (2007)
Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 23, 5108-16 (2005)
Nitz U, Gluz O, Kreipe HH, Christgen M, Kuemmel S, Baehner FL, et al. The run-in phase of the prospective WSG-ADAPT HR+/HER2- trial demonstrates the feasibility of a study design combining static and dynamic biomarker assessments for individualized therapy in early breast cancer. Ther Adv Med Oncol 12, 1758835920973130 (2020)
Nitz U, Gluz O, Kuemmel S, Christgen M, Braun M, Aktas B, et al. Endocrine therapy response and 21-gene expression assay for therapy guidance in HR-positive/HER2-negative early breast cancer. J Clin Oncol In press (2022)
Grote I, Bartels S, Kandt L, Bollmann L, Christgen H, Gronewold M, et al. TP53 mutations are associated with primary endocrine resistance in luminal early breast cancer. Cancer Med 10, 8581-94 (2021)
Hofmann D, Nitz U, Gluz O, Kates RE, Schinkoethe T, Staib P, et al. WSG ADAPT - adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trial. Trials 14, 261 (2013)
Lakhani SR, Ellis I, Schnitt S, Tan PH, van de Vijver M. (2012) WHO Classification of Tumours of the Breast. Lyon: International Agency for Research on Cancer.
Christgen M, Kandt LD, Antonopoulos W, Bartels S, Van Bockstal MR, Bredt M, et al. Inter-observer agreement for the histological diagnosis of invasive lobular breast carcinoma. J Pathol Clin Res 8, 191-205 (2022)
Parry S, Dowsett M, Dodson A UK NEQAS ICC & ISH Ki-67 Data Reveal Differences in Performance of Primary Antibody Clones. Appl Immunohistochem Mol Morphol 29, 86-94 (2021)
Viale G, Hanlon Newell AE, Walker E, Harlow G, Bai I, Russo L, et al. Ki-67 (30-9) scoring and differentiation of Luminal A- and Luminal B-like breast cancer subtypes. Breast Cancer Res Treat 178, 451-58 (2019)
Christgen M, von Ahsen S, Christgen H, Langer F, Kreipe H The region-of-interest size impacts on Ki67 quantification by computer-assisted image analysis in breast cancer. Hum Pathol 46, 1341-9 (2015)
Rimm DL, Leung SCY, McShane LM, Bai Y, Bane AL, Bartlett JMS, et al. An international multicenter study to evaluate reproducibility of automated scoring for assessment of Ki67 in breast cancer. Mod Pathol 32, 59-69 (2019)
Sahebjam S, Aloyz R, Pilavdzic D, Brisson ML, Ferrario C, Bouganim N, et al. Ki 67 is a major, but not the sole determinant of Oncotype Dx recurrence score. Br J Cancer 105, 1342-5 (2011)
Christgen M, Gluz O, Harbeck N, Kates RE, Raap M, Christgen H, et al. Differential impact of prognostic parameters in hormone receptor-positive lobular breast cancer. Cancer 126, 4847-58 (2020)
Robichaux JP, Elamin YY, Vijayan RSK, Nilsson MB, Hu L, He J, et al. Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity. Cancer Cell 36, 444-57 e7 (2019)
Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486, 353-60 (2012)
Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 163, 506-19 (2015)
Rinaldi J, Sokol ES, Hartmaier RJ, Trabucco SE, Frampton GM, Goldberg ME, et al. The genomic landscape of metastatic breast cancer: Insights from 11,000 tumors. PLoS One 15, e0231999 (2020)
Acknowledgements
We would like to thank Luisa Rieger and Laura Bollmann for excellent technical assistance.
Funding
The study was supported by a grant from the German Cancer Aid to S.B., M.C., O.G., N.H., and H.K. (Deutsche Krebshilfe, Grant Number 70112954). The funding body had no role in the design, data analysis, or manuscript preparation/publication. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
H.K., N.H., and M.C. designed the study. I.G., S.B., H.C. and L.K. performed the experiments, analyzed, and interpreted the data. M.R., M.G., M.R., and M.C. performed the immunohistochemical stainings and pathology assessment. O.G., M.G., S.K. and U.N. collected patient samples and data. I.G., H.K. and M.C. wrote the manuscript. U.L., O.G., N.H. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
O.G. has minority ownership interest in WSG GmbH, received honoraria from Genomic Health/Exact Sciences, Roche, Celgene, Pfizer, Novartis, NanoString Technologies, AstraZeneca, served in consulting/advisory role for Celgene, Genomic Health/Exact Sciences, Lilly, MSD, Novartis, Pfizer, Roche, and received travel support from Roche. SK has minority ownership interest in WSG GmbH, received personal fees from Lilly, Roche, Genomic Health, Novartis, Amgen, Celgene, Daiichi Sankyo, AstraZeneca, SOMATEX Medical Technologies, MSD, Pfizer, Puma Biotechnology, PFM medical, and non-financial support from Roche, Daiichi Sankyo, Sonoscope. U.N. has minority ownership interest in WSG GmbH, received honoraria from Agendia, Amgen, Celgene, Genomic Health, NanoString Technologies, Novartis pharma, Pfizer Pharmaceuticals, Roche/Genentech, Teva, served in consulting/advisory role for Genomic Health, Roche, provided expert testimony for Genomic Health, received travel support from Genomic Health, Pfizer Pharmaceuticals, Roche, and her institution received research funding from Agendia, Amgen, Celgene, Genomic Health, NanoString Technologies, Roche, Sanofi. N.H. has minority ownership interest in WSG GmbH, received honoraria from Amgen, AstraZeneca, Genomic Health, Novartis, Pfizer, Pierre Fabre, Roche, Zodiac Pharma, served in consulting/advisory role for Agendia, AstraZeneca, Celgene, Daiichi Sankyo, Lilly, Merck Sharp & Dohme, Novartis, Odonate Therapeutics, Pfizer, Pierre Fabre, Roche/Genentech, Sandoz, Seattle Genetics, an immediate family member served in consulting/advisory role for West German Study Group, and her institution received research funding from Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche/Genentech. I.G., S.B,. H.C,. M.R., M.G., L.K., M.R., U.L., M.G., M.C., and H.K., declare that they have no competing interests.
Ethics Approval and Consent to Participate
The study design is following the guidelines of the local ethics committee (“Ethics committee of the Medical School Hannover, ID 2716–2015”).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grote, I., Bartels, S., Christgen, H. et al. ERBB2 mutation is associated with sustained tumor cell proliferation after short-term preoperative endocrine therapy in early lobular breast cancer. Mod Pathol 35, 1804–1811 (2022). https://doi.org/10.1038/s41379-022-01130-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01130-7
This article is cited by
-
Zweitmeinungs- und Referenzpathologie beim Mammakarzinom
Die Pathologie (2022)