Abstract
Programmed cell death ligand 1 (PD-L1) on tumor cells is a significant prognostic biomarker for a number of malignancies, although less is known about the significance of PD-L1 positive immune cells in colon carcinoma. The purpose of this study is to evaluate the role of PD-L1 in a large cohort of colon carcinomas to identify patterns of PD-L1 expression in the tumor microenvironment and its correlation with other key immune subsets to better understand the impact of these immune cells. We assessed 1218 colon carcinomas on representative tissue microarray sections, gathered relevant clinicopathologic information, and performed immunohistochemical staining for mismatch repair proteins, CD8, CD163, LAG3, PD-L1, FoxP3, and BRAF V600E. We then performed automated quantification; manual quantification was used for PD-L1 tumor cells and immune cells. Dual PD-L1/PU.1 immunostain was also performed. The majority of PD-L1 positive cells expressed PU.1 thus representing tumor-associated macrophages. Based on the median number of PD-L1 positive immune cells (7.6/mm2), we classified tumors into two classes: (1) PD-L1 immune cell low and (2) PD-L1 immune cell high. PD-L1 immune cell high colon carcinomas showed favorable prognostic pathologic features including less frequent extramural venous invasion (p = 0.0001) and lower AJCC stage (p = 0.0001); they were also more commonly associated with deficient mismatch repair (dMMR) (p = 0.0001) and BRAF V600E reactivity. PD-LI immune cell high tumors were associated with high CD8, CD163, and FoxP3 positive cells (p = 0.0001, respectively). PD-L1 immune cell high and LAG3 high colon carcinomas were associated with improved disease-specific survival (p = 0.0001 and 0.001, respectively). PD-L1 expression on tumor cells was not associated with disease-specific survival. On multivariate analysis of chemotherapy naïve stage 2 colon carcinomas, only extramural venous invasion (p = 0.002), perineural invasion (p = 0.001) and PD-L1 immune cell expression (p = 0.032) correlated with disease-specific survival. Resected colonic carcinomas with high expression of PD-L1 and LAG3 proteins on immune cells were associated with improved prognosis in colon carcinoma. The mechanism underlying the improved prognosis of colon carcinomas bearing high numbers of immunoregulatory cells needs further investigation.
Similar content being viewed by others

Introduction
Immune checkpoint inhibitor (ICI) therapy has emerged as first- and second-line treatment options for a range of malignancies including melanoma, non-small cell lung carcinoma, and gastrointestinal adenocarcinoma, among others1,2,3. The therapy exploits the tumor microenvironment that innately suppresses the host cytotoxic T-cell response. One such widely acknowledged pathway of immune suppression involves overexpression of programmed cell death ligand-1 (PD-L1) in tumor cells and/or immune cells, which in turn negatively regulates host T-cells via the programmed cell death receptor (PD-1). Drugs that target the PD-L1/PD-1 axis enhance anti-tumor activity; as such, identifying pertinent histologic and immunologic features that predict this response may represent an independent prognostic variable and aid in predicting ICI response.
The focus in the gastrointestinal tract has been on the relationship between mismatch repair deficient (dMMR) tumors and response to ICI therapy. This concept is predicated on the idea that while tumors with a higher mutational burden express more neoantigens and elicit a greater host immune response4,5,6,7, dMMR tumors are also more likely to express immunosuppressive antigens, such as PD-L1 on tumors cells, as an adaptive measure to evade host immunity and render a less effective immune response8,9,10. Although there is merit in this concept, evidence to support this latter supposition is incomplete; dMMR tumors only demonstrate a response rate to ICI therapy in less than 40% of cases11,12. Additionally, there exists a subgroup of mismatch repair proficient (pMMR) patients, albeit a significantly smaller cohort, that has also shown a therapeutic response to ICI therapy, blurring the association with MMR status and necessitating the need for more predictive biomarkers11,13.
Several studies have demonstrated that PD-L1 expression on tumor cells is infrequent in colon cancer, whereas the expression of PD-L1 and PD-1 on intratumoral and peritumoral immune cells may be more common13,14,15,16. There is conflicting data in the literature regarding the respective impact of PD-L1 expression in tumor cells versus host immune cells on response to ICI therapy and prognosis. While some studies have advocated that PD-L1 expression on tumor-associated immune cells (namely myeloid cells) is more significant16,17,18, others argue that the expression of PD-L1 on tumor cells is more predictive of ICI response and prognosis8,9,19,20. Moreover, many studies focus on high-stage tumors and do not account for the confounding impact that neoadjuvant and adjuvant chemotherapy may have on the immune microenvironment, tumor biology, and PD-L1 expression, further blurring our understanding of the efficacy of PD-L1 as a prognostic biomarker21,22. Similarly, the role and expression pattern of other immune regulatory proteins such as LAG3, including its association with PD-L1 and as an independent biomarker further complicate the role of PD-L1. Nevertheless, the PD-L1 combined positive score is used as a predictive factor in gastric and gastroesophageal adenocarcinomas in routine practice.
The purpose of this study is to evaluate the role of PD-L1 positive immune cells in a large cohort of colon cancer and identify whether there exists a predictable pattern of PD-L1 expression in the tumor microenvironment, a correlation with other key immune subsets, and a prognostic significance to these cells.
Methods
We evaluated consecutive patients with colon adenocarcinomas resected (n = 1218) at the Massachusetts General Hospital. We excluded neuroendocrine carcinomas. The study was approved by MGB IRB.
Morphological and immunohistochemistry analysis
Tumor grading was performed as per WHO guidelines (low grade >50% vs. high grade ≤50% glandular area). We recorded the AJCC stage, perineural invasion, and extramural venous invasion. We also recorded MMR status based on immunohistochemistry for MSH2, MSH6, MLH1, and PMS2.
Immunohistochemistry was performed on tissue microarrays. The central portion of each tumor was represented by a 0.2 cm core of tissue. We specifically avoided areas with mucin. Detailed information about the clone, dilution, type of antibody, and company are listed in supplementary table 1. Immunohistochemistry for BRAF V600E, MMR proteins, and the PD-L1/PU.1 dual stain was performed on the Leica Bond. We have previously demonstrated high concordance between BRAF V600E immunohistochemistry and BRAF sequencing23. Immunohistochemistry for CD8, PU.1, CD163, PD-L1, LAG3, and FoxP3 was performed following heat-induced epitope-retrieval (HIER). The sections were incubated in 10 mM sodium-citrate (pH6.0) or 10 mM Tris (pH9.0) buffered solutions containing 0.05% Tween at 125 °C for 5 min using a decloaking chamber (Biocare Medica). Staining was performed on the automated LabVision automated 360 (Thermo Scientific) platform using a secondary ImmPRESS polymer detection system (Vector Laboratories) and horseradish-peroxidase-conjugated rabbit anti-sheep secondary antibodies (Thermo Scientific), according to the manufacturers protocols. The Vulcan Fast Red Chromogen Kit 2 (red staining; Biocare Medical) or the DAB Quanto System (brown staining; Thermo Scientific) were applied as substrates. With every batch, a known tissue-positive control was used.
Automated quantification
The stained TMA slides were scanned using Aperio ScanScope digital slide scanner (Aperio ScanScope CSO, Leica Biosystems Imaging, CA). Automated quantification of cells positive for CD8, CD163, LAG3, and FoxP3 was performed using the HALO image analysis platform (HALO 2.3; Indica Laboratories, NM) on the entire core of tissue. Tumor area within each tissue core was manually annotated. The number of positive CD8, CD163 and FoxP3 cells in the entire available tissue were calculated and expressed per mm2.
Manual quantification (Semiquantitative)
The percentage of tumor cells staining for PD-L1 was recorded in increments of 5% on the TMA slides. We also manually quantitated the number of intratumoral PD-L1 positive inflammatory cells on the entire core of tissue, and the results were expressed per mm2. The latter quantification was performed on scanned slides with individual cells marked using Aperio ScanScope. The number of intratumoral inflammatory cells positive for PD-L1 alone and dual positive for PD-L1 and PU.1 were manually quantitated in 20 PD-L1 high colon cancers in a select 0.238 mm2 field.
Validation of PD-L1 on whole sections
We evaluated whole sections on 16 cases from this cohort, 11 from the low end of the spectrum and 5 from the high end of the spectrum. Representative whole sections from the select cases were scanned using Aperio ScanScope digital slide scanner. On each whole section, we manually annotated the number of intratumoral inflammatory cells positive for PD-L1 both in 5 contiguous fields in the center of the tumor and 5 contiguous fields at the most infiltrative front of the tumor and values at 40x magnification and the average value per case was expressed per mm2.
Statistical analysis
Follow-up duration was calculated from the time of operation to the time of death or last follow-up. Survival curves were plotted using the Kaplan-Meier method. Differences in disease-specific survival between groups were analyzed by the log-rank test. Multivariate survival analyses were performed using the backward conditional Cox regression method. Comparison of categorical variables among groups was performed using chi- square test or Fisher exact test. Independent sample T-test and Pair-sample t-test were used where appropriate. All analyses were performed using SPSS version 21. A p-value of <0.05 was considered statistically significant.
Results
Description of the cohort
The study included 1218 resected colonic adenocarcinomas. 49.8% (607/1218) of patients were female. The mean age at diagnosis was 66.5 years (range 23->90). There were 14 patients with Lynch syndrome and 2 patients with familial adenomatous polyposis syndrome.
All 4 AJCC stages were represented in this cohort: stage I-227 (18.7%), stage II-409 (33.6%), stage III 430 (35.4%), and stage IV 143 (11.8%). 14.4% (175/1214) of patients were treated with neoadjuvant therapy. Staging information was not available in 9 cases. Adjuvant therapy was used in 44.8% of patients.
PD-L1 positive immune cells
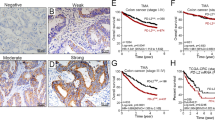
Information on PD-L1 immune cells was available in 1039 cases. The median number of PD-L1 positive immune cells was 7.6/mm2 (mean 46.2, SD 97.8 (range 0–318.4)). For the purposes of this study, we used the median value to stratify patients into 2 groups: PD-L1 immune cell low (Fig. 1A, B) and PD-L1 immune cell high (Fig. 1C, D).
A Colon carcinoma with few associated intratumoral inflammatory cells and the corresponding B immunostain for PD-L1 is negative in both the intratumoral inflammatory cells and tumor cells. In contrast, C this colon carcinoma demonstrates numerous intratumoral inflammatory cells with a corresponding D PD-L1 high immunophenotype revealing intense red membranous staining in the peritumoral inflammatory component.
PD-L1/PU.1 dual stain
Twenty PD-L1 immune cell high tumors were selected for dual PD-L1/PU.1 stain. The mean ratio of cells staining for both markers to the total number of PD-L1 positive cells was 0.70 (range from 0.60 to 0.78), indicating that the majority of the PD-L1 positive cells in the immune microenvironment are tumor-associated macrophages (Fig. 2A, B). Of note, as highlighted in low immune PD-L1 tumors (Fig. 2C), neutrophils reacting with PU.1 were excluded in this quantification by identification of their distinctive nuclear features.
A Dual stain for PU.1 and PD-L1 of a PD-L1 immune high colon cancer reveals the majority of PD-L1 positive (brown membranous staining) immune cells are also positive for PU.1 (macrophages, red nuclear chromogen). This is further highlighted in the B lumen of a malignant gland. In contrast, C dual stain in a PD-L1 low immune colon cancer reveals background macrophages and neutrophils with no PD-L1 membrane signal.
Correlation of PD-L1 immune cell high carcinomas with clinical, pathologic, and molecular features
There was a strong correlation between the number of PD-L1 positive immune cells and key clinical, pathologic, and molecular features (Table 1). Patients classified as PD-L1 immune cell high were associated with lower AJCC stage, a pushing (rather than infiltrative) tumor border, and less likely to show extramural venous invasion. PD-L1 immune cell high tumors were more likely to be dMMR and positive for BRAF V600E on immunohistochemistry. A similar correlation was noted when we added patients treated with neoadjuvant therapy (supplementary table 2). There was no association seen with gender or tumor location, and the PD-L1 immune high tumors trended more common in older patients and show less frequent perineural invasion.
Impact of PD-L1 immune cell high on disease-specific survival
PD-L1 immune cell high tumors were associated with longer disease-specific survival than PD-L1 immune cell low tumors (Fig. 3A). In the entire cohort, the mean estimated survival for PD-L1 immune cell high tumors was 137.5 months (range 123.6 to 132.8) while that for PD-L1 immune cell low tumors was 116.9 months (range 109.6 to 124.2) (p = 0.0001). PD-L1 immune cell high patients showed improved disease-specific survival in both pMMR tumors (p = 0.0001) and dMMR tumors (p = 0.028).
Kaplan Meier curve for disease-specific survival (in months) reveals survival benefit of PD-L1 immune high relative to PD-L1 low colon carcinomas for A all cases (p = 0.0001), B for cases receiving no neoadjuvant therapy (p = 0.0001), and C for stage 2 cases receiving no adjuvant therapy (p = 0.008).
Following the exclusion of patients treated with neoadjuvant therapy (n = 175), the mean estimated survival for PD-L1 immune cell high patients was 139.3 months (range 133.4 to 145.2) while that for PD-L1 immune cell low patients was 118.6 months (range 110.6 to 126.5) (p = 0.0001) (Fig. 3B).
After excluding AJCC stage 2 patients treated with neoadjuvant therapy (n = 357), we found that the mean estimated survival for PD-L1 immune cell high patients was 158.1 months (range 142.7 to 156.2) while that of PD-L1 immune cell low patients was 138.8 months (range 126.9 to 150.6) (p = 0.008). A similar result was obtained in stage 2 tumors following the additional exclusion of patients treated with adjuvant (as well as neoadjuvant) therapy. The mean estimated survival for PD-L1 immune cell high was 161.3 months (range 154.6 to 168) while that for PD-L1 immune cell low patients was 140.5 months (range 127.4 to 153.7) (p = 0.008) (Fig. 3C). Of note, PD-L1 on immune cells did not correlate with disease-specific survival in stage 3 or stage 4 tumors (Table 2).
Multivariate analysis
On univariate analysis, including lower AJCC stage (p = 0.0001), absence of extramural venous (p = 0.0001) and perineural invasion (p = 0.0001), lower tumor grade (p = 0.0001), and higher number of CD8 cells (p = 0.0001) correlated with improved disease-specific survival. On a multivariate analysis (after excluding patients treated with neoadjuvant therapy) that included clinical and pathologic features that correlated significantly with survival only AJCC stage, extramural venous invasion and perineural invasion correlated with disease-specific survival (Table 3). On a multivariate analysis comprised of only stage 2 colon adenocarcinomas that did not receive adjuvant therapy, only absence of extramural venous invasion, perineural invasion, and PD-L1 immune cell high cases correlated with improved disease-specific survival.
Validation of PD-L1 on whole sections
To validate the results of the tissue microarray we performed PD-L1 stain on whole sections in 16 cases, 5 with high PD-L1 scores, and 11 with low PD-L1 scores. An assessment of whole slide PD-L1 sections revealed that the reactivity in the immune compartment, when present, was localized to the interface of the broad stromal septa and tumor (Fig. 4) (supplementary fig. 1). Thus, although PD-L1 reactivity was more prevalent at the advancing front of the tumor, a dense peritumoral inflammatory infiltrate also extended into the central portion of the tumor.
Immunohistochemistry showing PD-L1 high (A) and PD-L1 low (B) colon carcinoma. The PD-L1 high cases showed diffuse staining in the immune compartment at the tumor stromal interface (A). The PD-L1 low cases were either entirely negative as seen in panel B, or showed rare pockets of positive immune cells.
There was a strong correlation between the counts performed on the microarray and whole sections (correlation 0.71). Additionally, on a paired sample t test there was no difference between the two methods (p = 0.9).
PD-L1 on tumor cells
We also semi-quantitively evaluated PD-L1 reactivity on tumor cells. 19.6% of tumors showed reactivity in >1% of tumor cells and 17.6% showed reactivity in >5% of tumor cells (Table 4). The mean reactivity was 1.1% (range 0-100). PD-L1 tumor scores of >1% and >5% cut points were more likely to be dMMR (p = 0.0001, p = 0.0001, respectively). PD-L1 reactivity at >1% cut point or >5% cut point did not correlate with disease specific survival (p = 0.4, p = 0.67, respectively). Notably, PD-L1 expression on tumor cells did not correlate with PD-L1 expression on immune cells (p = 0.265).
Correlation of PD-L1 immune cells with other immune cells
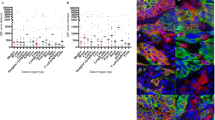
The PD-L1 immune high cohort was associated with increased numbers of CD8 positive cells (p = 0.0001), FoxP3 positive cells (p = 0.0001), CD163 positive cells (p = 0.001), and LAG3 positive cells (p = 0.21), although the latter did not achieve significance.(Supplementary table 3) (Figs. 5 and 6).
PD-L1 immune high colon cancer reveals an inflamed tumor microenvironment (A) that demonstrates a strong positive correlation between PD-L1 immune cells (D), with inset highlighting red membranous staining, CD8 positive T-lymphocytes (B), PU.1 positive tumor-associated macrophages (C), and LAG3 positive immune cells (E).
PD-L1 immune low colon carcinoma reveals a pauci-immune tumor microenvironment (A) that demonstrates a strong positive correlation when there is a negative/low signal for PD-L1 (D), with few CD8 positive T-lymphocytes (B), no/rare PU.1 positive tumor-associated macrophages (C), and no/rare LAG3 positive (E) immune cells.
Relationship between LAG3 and CD163 and survival
Based on the median number of LAG3 positive cells (2.5/mm2), patients with a high number of LAG3 positive cells (>greater than median) showed significantly better survival than those with a low number of cells (p = 0.001) (Fig. 7). Notably, the number of CD163 positive cells, stratified by the median number of CD163 positive cells, did not correlate with disease-specific survival (p = 0.22).
Discussion
PD-L1 expression is currently not used in predicting efficacy of ICI therapy in colon carcinoma, and there are no clear-cut guidelines regarding its interpretation, utilization, and significance. In this study, we sought to investigate the pattern of PD-L1 expression in colon carcinoma for two distinct cellular compartments, tumor, and immune cells, and the relationship this pattern has with other elements of the tumor immune microenvironment. Herein, we report that PD-L1 expression in host immune cells, largely tumor-associated macrophages, predicts favorable outcome measures, although the effect is most pronounced in stage 2 tumors. Similarly, patients with high numbers of LAG3 positive cells also showed improved disease-specific survival. We found that the expression of PD-L1 on host immune cells correlated positively with the number of CD8 positive tumor-infiltrating lymphocytes and FoxP3 positive cells, the latter underlining a nuanced tumor immune microenvironment that counterintuitively upregulates membranous proteins known to quell the otherwise favorable immune response. Lastly, we found no significant association of tumor PD-L1 expression with objective outcome measures or immune cell PD-L1 expression.
In this study, we found that PD-L1 immune cell high tumors are associated with a lower AJCC stage, less frequent extramural venous invasion, and favorable disease-free survival. In the only study that systematically evaluated the prognostic impact of PD-L1 immune cells in colon carcinoma, Miller et al. evaluated stage 3 colon carcinomas and identified older age at surgery, lower histologic grade, and PD-L1 positive CD11c myeloid cells as the only statistically significant parameters that correlated with overall survival, and remarkably this benefit was independent of CD824. Nonetheless, this study focused exclusively on stage 3 tumors, and the authors were unable to account for the confounding effect of chemotherapy. It is notable that in our study, the improved survival of the PD-L1 immune cell high cohort was most apparent in stage 2 colon carcinomas that did not receive adjuvant or neoadjuvant chemotherapy.
Similar efforts to characterize PD-L1 expressing host immune cells in non-small cell lung carcinoma (NSCLC) yielded comparable results. Liu and colleagues demonstrated that PD-L1 co-localization was overwhelmingly associated with macrophages in the stroma and was the predominant immune cell co-localizing PD-L1 in the tumor. Furthermore, other than CD8, they found this biomarker to be the only significant prognosticator associated with better overall survival in patients treated with immunotherapy25. While it is hard to conceptually accept the notion that macrophage expression of PD-L1 is associated with a favorable prognosis, particularly since it is well established to be a known immunosuppressant and negative prognosticator when expressed by NSCLC tumor cells, a number of studies have generated explanations to explain this correlative epiphenomenon. It is reported in early NSCLC26, tumor-associated macrophages often co-express stimulatory and inhibitory proteins (including PD-L1), and there was no significant tendency of these cells to express more inhibitory or stimulatory markers and no significant link of PD-L1 on tumor-associated macrophages to T-cell function. While the focus the current study was on inhibitory signals, we did not assess for potential opposing stimulatory signal, which may have accounted for this ‘counterintuitive’ data i.e. the favorable effect of PD-L1 immune cells. Other studies have suggested that PD-L1 expression by tumor-associated macrophages may be a defensive measure to protect themselves from CD8+ T-cell induced cytolysis and in turn protect the immune stimulatory function of these cells, including antigen-presenting cells, suggesting an alternative ‘pro-stimulatory’ mechanism of PD- L126,27. Collectively, while the inherent tumor biology of NSCLC is different than colon cancer, a number of parallels can be drawn between their host immune microenvironments, and it is fair to speculate that the role of PD-L1 positive macrophages have comparable roles, although further studies are required to elucidate this mechanism.
The current study found that LAG3, another immune regulatory protein28, corroborates prior studies that have demonstrated that LAG3 expression on CD8+ T-cells to be associated with longer progression-free survival in high-stage colon cancer29. Indeed, the utility of LAG3 as a biomarker and an actionable target, particularly as combination therapy with other checkpoint inhibitors, is gaining therapeutic steam as current trials are demonstrating beneficial outcomes in a hard-to-treat pMMR metastatic colon carcinoma30,31.
It is widely accepted that CD8 positive T-cells are a favorable prognosticator in colon cancer, indicating that host immune response against the tumor portends a more favorable outcome32,33. It is speculated that in tumors that generate a strong CD8 T-cell response, upregulation of PD-L1 expression in antigen-presenting cells is mediated by pro-inflammatory cytokines, most characteristically interferon-gamma, in an attempt to curtail the exuberant activity of T-cells34,35,36,37. These PD-L1 positive antigen-presenting cells maintain self-tolerance by binding the activated T-cells at their PD-1 receptor and inducing an inhibitory/deactivating signal34,35,36,37. As such, the favorable prognosis of PD-L1 immune cell high tumors identified in this study would appear counterintuitive since theoretically these cells would correlate with an exhausted and deactivated T-cell population. An alternative model, though highly speculative, would argue that the PD-L1 tumor-associated macrophages and FoxP3 positive regulatory lymphocytes are simply a surrogate marker of a hyperimmune response to the tumor. The current study supports this conjecture as both CD163 (p = 0.001) and FoxP3 (p = 0.0001) were positively associated with PD-L1 high immune high tumors and are part of an evolving view that immune biomarkers should be interpreted based on the overall context of the immune microenvironment. PD-L1 positive immune cells and FoxP3 positive regulatory T-cells, which are typically associated with immune evasion, can, therefore, have a seemingly paradoxically good prognosis38,39.
The significance of PD-L1 expression in colon tumor carcinoma is perhaps even more controversial than host immune cells and continues to be debated. Conceptually, one would expect that the expression of PD-L1 on tumor cells would impart an aggressive phenotype by allowing the cancer cells to evade immunity and exert an inhibitory function on host immune cells. While this appears to be a significant negative prognosticator for a number of malignancies40,41, multiple studies have demonstrated conflicting results in colonic carcinomas, with little consensus on its prognostic value. Surprisingly, some studies demonstrate that tumor cell PD-L1 expression is associated with improved survival42, while other studies have shown decreased survival43. We found that >20% of tumors (both at >1% and >5% cut point) demonstrate membranous PD-L1positivity and this pattern was seen more commonly seen in dMMR tumors; however, this expression did not reach significance on disease-specific survival for both the entire cohort and subset cohorts based on tumor stage and/or chemotherapy status. In fact, this appears to be in line with an evolving consensus that favors the view that PD-L1 expression on tumor cells does not impact outcome13,14. Nevertheless, there are a number of explanations that can underline the discrepancies and limitations of tumor PD-L1 expression including intratumoral heterogeneity, varying antibody assays, differing PD-L1 cutoffs, small sample size, and the effect of neoadjuvant/adjuvant therapy.
The study also raises intriguing questions about a widely used scoring system for PD-L1, the combined positive score (CPS), for a variety of carcinomas including gastric and gastro-esophageal carcinoma, and breast, among others44. The numerator on this scoring system adds two potentially disparate markers, PD-L1 on tumor cells and PD-L1 on immune cells and notably, the number of positive immune cells often overshadow positive tumor cells. The objective response rate for PD-1 inhibitors in gastric carcinomas was higher in tumors with a CPS score of ≥1% (15.5%) compared to patients with a score of <1% (6.4%)44. While speculative, we posit that since PD-L1 on immune cells has not been critically evaluated in gastric carcinoma, the potential improved outcome of patients with high CPS may reflect the innately better survival of this group of tumors.
Our study has multiple limitations. Given that the study was performed on tissue microarrays, we do not account for the heterogeneity typically associated with immune markers. However, the tissue microarray approach allows for the evaluation of multiple immunohistochemical markers in one of the largest cohorts systematically evaluated for immune markers. Additionally, we did evaluate for tumor heterogeneity on a subset of whole tissue sections for both PD-L1 high and PD-L1 low groups. Another limitation is that we are unable to correlate our findings with response to ICI therapy, which would be essential to guide therapeutic insight. Furthermore, our study focused entirely on inhibitory antigens including PD-L1 and LAG3, limiting our ability to elaborate on the larger context and situation in which these markers are expressed.
In conclusion, we evaluate a large cohort of resected colon carcinoma and assess the impact of PD-L1 reactivity on immune cells to draw the following broad conclusions:
(1) High PD-L1 and LAG3 expression on immune cells is associated with improved disease-free survival; (2) PD-L1 expression on tumor cells is not a prognostic variable, and (3) the PD-L1 immune cell high cohort was associated with increased numbers of CD8+ T-cells as well as CD163+ and FoxP3+ cells, arguing for a coordinated underlying mechanisms that accounts for these immunologically active tumors. The improved prognosis associated with PD-L1 and LAG3 high tumors appears counterintuitive, given the immunosuppressive nature of these cells, and we hypothesize that the role of this phenotype is part of a larger and more intricate tumor microenvironment that should not be broadly labeled/stigmatized as inhibitory or stimulatory based on their PD-L1 expression status. Future directions will seek to elucidate a more comprehensive evaluation of regulatory and stimulatory immune biomarkers on tumor cells and host immune cells that might shed light on the role and utility of PD-L1 in the tumor microenvironment and whether ICI efficacy is associated with a particular phenotype.
Data availability
The data that support the findings of this study are not openly available to maintain patient confidentiality but de-identified data are available from the corresponding author upon reasonable request.
References
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Taieb J, Moehler M, Boku N, Ajani JA, Yanez Ruiz E, Ryu MH, et al. Evolution of checkpoint inhibitors for the treatment of metastatic gastric cancers: Current status and future perspectives. Cancer Treat Rev. 66, 104–113 (2018).
Thota R, Gonzalez RS, Berlin J, Cardin DB, Shi C. Could the PD-1 pathway be a potential target for treating small intestinal adenocarcinoma? Am J Clin Pathol. 148, 208–214 (2017).
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 17, 129 (2018).
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 366, 2455–2465 (2012).
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 366, 2443–2454 (2012).
Noguchi T, Ward JP, Gubin MM, Arthur CD, Lee SH, Hundal J, et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res. 5, 106–117 (2017).
Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. 8, 14572 (2017).
Lee LH, Cavalcanti MS, Segal NH, Hechtman JF, Weiser MR, Smith JJ, et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 29, 1433–1442 (2016).
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 372, 2509–2520 (2015).
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191 (2017).
Liu S, Gnen M, Stadler ZK, Weiser MR, Hechtman JF, Vaklani E, et al. Cellular localization of PD-L1 expression in mismatch-repair-deficient and proficient colorectal carcinomas. Mod Pathol. 32, 110–121 (2019).
Eriksen AC, Sorensen FB, Lindebjerg J, Hager H, dePont Christensen R, Kjaer-Frifeldt S, et al. Programmed Death Ligand-1 expression in stage II colon cancer - experiences from a nationwide populationbased cohort. BMC Cancer. 19, 142 (2019).
Wang L, Liu Z, Fisher KW, Ren F, Lv J, Davidson DD, et al. Prognostic value of programmed death ligand 1, p53, and Ki-67 in patients with advanced-stage colorectal cancer. Hum Pathol. 71, 20–29 (2018).
Yomoda T, Sudo T, Kawahara A, Shigaki T, Shimomura S, Tajiri K, et al. The immunoscore is a superior prognostic tool in Stages II and III colorectal cancer and is significantly correlated with Programmed Death-Ligand 1 (PD-L1) expression on tumor-infiltrating mononuclear cells. Ann Surg Oncol. 26, 415–424 (2019).
Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest. 128, 580–588 (2018).
Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest. 128, 1708 (2018).
Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 214, 895–904 (2017).
Kleinovink JW, Marijt KA, Schoonderwoerd MJA, van Hall T, Ossendorp F, Fransen MF. PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology. 6, e1294299 (2017).
Pol J, Buque A, Aranda F, Bloy N, Cremer I, Eggermont A, et al. Trial Watch-Oncolytic viruses and cancer therapy. Oncoimmunology 5, e1117740 (2016).
Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 45, 1470-1476 (2008).
Routhier CA, Mochel MC, Lynch K, Dias-Santagata D, Louis DN, Hoang MP. Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAF V600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Hum Pathol. 44, 2563–2570 (2013).
Miller TJ, Anyaegbu CC, Lee-Pullen TF, Spalding LJ, Platell CF, McCoy MJ. PD-L1+ dendritic cells in the tumor microenvironment correlate with good prognosis and CD8+ T cell infiltration in colon cancer. Cancer Sci. 112, 1173–1183 (2021).
Liu Y, Zugazagoitia J, Ahmed FS, Henick BS, Gettinger SN, Herbst RS, et al. Immune Cell PD-L1 Colocalizes with Macrophages and Is Associated with Outcome in PD-1 Pathway Blockade Therapy. Clin Cancer Res. 26, 970–977 (2020).
Singhal S, Stadanlick J, Annunziata MJ, Rao AS, Bhojnagarwala PS, O’Brien S, et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med. 11 (2019).
Mueller SN, Jones CM, Stock AT, Suter M, Heath WR, Carbone FR. CD4+ T cells can protect APC from CTL-mediated elimination. J Immunol. 176, 7379–7384 (2006).
Huard B, Tournier M, Hercend T, Triebel F, Faure F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol. 24, 3216–3221 (1994).
Zhou G, Noordam L, Sprengers D, Doukas M, Boor PPC, van Beek AA, et al. Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology 7, e1448332 (2018).
Papadopoulos KP, Johnson ML, Lockhart AC, Moore K, Falchook GS, Formenti SC, et al. First-in-human study of cemiplimab alone or in combination with radiotherapy and/or low-dose cyclophosphamide in patients with advanced malignancies. Clin Cancer Res. 26, 1025-1033 (2020).
Harding JJ, Zhu AX, Bauer TM, Choueiri TK, Drilon A, Voss MH, et al. A Phase Ib/II Study of Ramucirumab in combination with emibetuzumab in patients with advanced cancer. Clin Cancer Res 25, 5202-5211 (2019).
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960-1964 (2006).
Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 67, 1883–1886 (2007).
Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med. 375, 1767–1778 (2016).
Okazaki T, Honjo T. PD-1, and PD-1 ligands: from discovery to clinical application. Int Immunol. 19, 813–824 (2007).
Francisco LM., Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 236, 219–242 (2010).
Chiu YM, Tsai CL, Kao JT, Hsieh CT, Shieh DC, Lee YJ, et al. PD-1 and PD-L1 up-regulation promotes T-cell apoptosis in gastric adenocarcinoma. Anticancer Res. 38, 2069-2078 (2018).
Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 60, 909–918 (2011).
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 27, 186-192 (2009).
Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 25, 1935-1940 (2014).
Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 8, 328rv324 (2016).
Huang CY, Chiang SF, Ke TW, Chen TW, You YS, Chen WT et al. Clinical significance of programmed death 1 ligand-1 (CD274/PD-L1) and intra-tumoral CD8+ T-cell infiltration in stage II-III colorectal cancer. Sci Rep. 8, 15658 (2018).
Enkhbat T, Nishi M, Takasu C, Yoshikawa K, Jun H, Tokunaga T, et al. Programmed cell death Ligand 1 expression is an independent prognostic factor in colorectal cancer. Anticancer Res. 38, 3367-3373 (2018).
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Macahdo M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 Clinical KEYNOTE-059 trial. JAMA Oncol. 4, e180013 (2018).
Funding
Vikram Deshpande is partially funded by NIH grant.
Author information
Authors and Affiliations
Contributions
Contributed to collection of material, planning and performing experiments and analysis of data: AN, AP, AC, LL, AD, MT, SGS, RC, MLZ, OHY, SR. Conceived and planned the experiments: VD, DTT, DB, SC, QZ, MT, SGS, RC, MLZ, OY, DP. Conceived the study and in charge of overall direction and planning: VD. Wrote the paper with input from all authors: AN, AP, AC, LL, AD, MT, SGS, RC, MLZ, OHY, SR, ERL, QZ, SC, OHY, OY, CF, DTP, DTT, DB, VD. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
DTT has received consulting fees from ROME Therapeutics, Tekla Capital, Ikena Oncology, Foundation Medicine, Inc., NanoString Technologies, EMD Millipore Sigma, and Pfizer that are not related to this work. DTT is a founder and has equity in ROME Therapeutics, PanTher Therapeutics, and TellBio, Inc., which is not related to this work. DTT receives research support from ACD-Biotechne, PureTech Health LLC, and Ribon Therapeutics, which was not used in this work. DTT’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. The other authors do not have any relevant disclosures.
Ethics approval
The study was approved by MGB IRB number 2017P61.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yılmaz, O., Pankaj, A., Neyez, A. et al. Programmed death-ligand 1 expression in the immune compartment of colon carcinoma. Mod Pathol 35, 1740–1748 (2022). https://doi.org/10.1038/s41379-022-01128-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01128-1
This article is cited by
-
PU.1 negatively regulates tumorigenesis in non-small-cell lung cancer
Medical Oncology (2023)