Abstract
Rare cases of human herpesvirus 8 (HHV8)-negative effusion-based large B-cell lymphoma (EB-LBCL) occur in body cavities without antecedent or concurrent solid mass formation. In contrast to HHV8 + primary effusion lymphoma (PEL), EB-LBCL has no known association with HIV or HHV8 infection. However, the small sample sizes of case reports and series worldwide, especially from non-Japanese regions, have precluded diagnostic uniformity. Therefore, we conducted a retrospective, multi-institutional study of 55 cases of EB-LBCL and performed a comprehensive review of an additional 147 cases from the literature to identify distinct clinicopathologic characteristics. In our study, EB-LBCL primarily affected elderly (median age 80 years), immunocompetent patients and manifested as lymphomatous effusion without a solid component. The lymphomatous effusions mostly occurred in the pleural cavity (40/55, 73%), followed by the pericardial cavity (17/55, 31%). EB-LBCL expressed CD20 (53/54, 98%) and PAX5 (23/23, 100%). Most cases (30/36, 83%) were of non-germinal center B-cell subtype per the Hans algorithm. HHV8 infection was absent (0/55, 0%), while Epstein-Barr virus was detected in 6% (3/47). Clinically, some patients were managed with drainage alone (15/34, 44%), while others received rituximab alone (4/34, 12%) or chemotherapy (15/34, 44%). Eventually, 56% (22/39) died with a median overall survival (OS) of 14.9 months. Our findings were similar to those from the literature; however, compared to the non-Japanese cases, the Japanese cases had a significantly higher incidence of pericardial involvement, a higher rate of chemotherapy administration, and longer median OS. Particularly, we have found that Japanese residence, presence of pericardial effusion, and absence of MYC rearrangement are all favorable prognostic factors. Our data suggest that EB-LBCL portends a worse prognosis than previously reported, although select patients may be managed conservatively. Overall, EB-LBCL has distinct clinicopathologic characteristics, necessitating the establishment of separate diagnostic criteria and consensus nomenclature.
Similar content being viewed by others
Introduction
Cases of large B-cell lymphoma have been observed in the body cavities without associated tumor masses. A substantial proportion of these cases occur in human immunodeficiency virus (HIV)-positive patients and are associated with human herpervirus-8 (HHV8) infection, which has been recognized by the World Health Organization (WHO) Classification as a distinct entity known as primary effusion lymphoma (PEL)1,2. PEL is commonly positive for CD45, CD138, and CD30, while the pan B-cell markers CD20, CD79a and PAX5 are negative. In addition to HHV8 infection, most cases of PEL are positive for Epstein-Barr virus (EBV) infection. However, it has become increasingly apparent that HHV8-negative large B-cell lymphomas may occur in body cavities without lymphomatous mass formation3,4,5,6,7. Despite some similarities to PEL in clinical presentation and cytologic features, these HHV8-negative effusion-based large B-cell lymphomas (EB-LBCLs) typically occur in HIV-negative, elderly patients, and the lymphoma cells are usually positive for pan B-cell markers.
Currently, there is no standardized nomenclature for the EB-LBCL. In the English literature, it has been described as HHV8-negative PEL8,9,10, primary HHV8-negative effusion-based lymphoma5, HHV8-negative malignant effusion lymphoma11, PEL-like lymphoma12, primary PEL-like lymphoma13, HHV8-unrelated PEL-like lymphoma7,14,15,16, and type II PEL17. Although EB-LBCL and PEL have similar clinical presentations with lymphomatous effusions in the body cavities and no solid mass formation, they appear to be distinct entities as demonstrated by different demographic features, etiology, immunophenotype, and prognosis. Hence, using the nomenclature of “PEL” in this HHV8-negative entity may cause further confusion for treating physicians. In this article, we tentatively designate the HHV8-negative effusion lymphoma as “EB-LBCL”. The ultimate nomenclature of this HHV8-negative entity may be best held until there is consensus among hematopathology and hematology societies.
The 2017 WHO Classification has not defined EB-LBCL as a specific entity. It is currently uncertain as to whether EB-LBCL should be considered a unique entity or a neoplasm that exists along the clinical spectrum of conventional diffuse large B-cell lymphoma (DLBCL). Most cases of EB-LBCL were reported in Japan, though there were also scattered case reports and series elsewhere worldwide. According to the literature, the clinical behavior and prognosis of EB-LBCL are highly variable. Surprisingly, a small number of patients experience spontaneous resolution of disease after drainage of body fluids alone without chemotherapy18,19,20,21,22. We have also encountered similar cases in our practice. In addition, based on a large Japanese cohort, EB-LBCL was associated with a favorable prognosis5. However, from our own experience in the United States (US), many patients had a dismal prognosis.
EB-LBCL may pose a challenge to pathologists since it has no clear consensus terminology or diagnostic criteria, and it is therefore likely underappreciated in practice. In addition, the clinical behavior and optimal management of EB-LBCL remain to be further elucidated. Hence, we conducted this multi-institutional retrospective study in the US and present a comprehensive review of the literature worldwide to define the clinicopathologic features of EB-LBCL.
Materials and Methods
Case selection
The pathology archives from multiple institutions within the US were reviewed to identify cases of EB-LBCL from 2000 to 2022. The inclusion and exclusion criteria are summarized in Table 1. In brief, only HHV8-negative large B-cell lymphomas initially presenting as a malignant effusion without any solid tumor mass were selected for this study. In addition, effusion lymphomas with unknown HHV8 status, plasmablastic immunophenotype, history of low-grade B-cell lymphoma, or history of organ transplant were excluded from this study. Ultimately, a total of 55 cases of EB-LBCL were included, all of which had no antecedent or concurrent solid large B-cell lymphoma. The diagnosis of each case was confirmed with necessary clinical data, immunohistochemical stains, and molecular genetic studies.
An extensive literature search was performed in PubMed using a combination of different keywords, including “HHV8-negative”, “HHV8-unrelated”, “primary effusion lymphoma”, “effusion-based lymphoma”, “malignant effusion lymphoma”, and “primary effusion lymphoma-like (PEL-like)”. All published cases were carefully reviewed to exclude those with an ambiguous diagnosis or those with plasma cell myeloma or T-cell lymphoma. A total of 147 cases of EB-LBCL met our criteria and were retrieved from the literature4,5,6,7,9,10,11,12,14,15,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53, which included 64 cases from a large Japanese cohort (hereafter referred to as the “Kaji group”)5, 23 additional cases from Japan (hereafter referred to as the “non-Kaji group”), 20 East Asian cases from Korea and Taiwan (hereafter referred to as the “other East Asian group”), and 40 cases from the Western countries (hereafter referred to as the “Western group”). Based on the geographic and survival data (more information in Survival Analyses under Literature Review) as well as previous reports of Japanese ethnicity being a favorable prognostic factor54, the Kaji and non-Kaji groups were combined and referred to as the “Japanese group” in some of the statistical analyses. Likewise, the other East Asian and Western groups in the literature were combined and referred to as the “non-Japanese group”. The non-Japanese groups in the literature were combined with the cases from our study to form the “all-non-Japanese group”.
Specimen processing and cytologic evaluation
The cytology smears of the lymphomatous effusions were made into cell blocks by centrifuging the samples into pellets via traditional methods or using the Cellient™ Automated Cell Block Processor (Hologic Corporation, Marlborough, MA, USA). The samples were then sent to histology where they were subsequently sectioned at 4.0 μm and stained with hematoxylin & eosin (H&E). The cytologic features of lymphoma cells were classified into: 1) Centroblastic: large cells with round nuclei, vesicular chromatin, and several small peripheral nucleoli; 2) Immunoblastic: large cells with round and centrally located nuclei, vesicular chromatin and a prominent, eosinophilic nucleolus; 3) Plasmablastic: large cells with round, often eccentrically placed nuclei with one or several prominent nucleoli; and 4) Pleomorphic: large or anaplastic cells with variable sizes and shapes, frequent multinucleation, and prominent nucleoli.
Immunohistochemical staining
Immunohistochemical stains were performed on the cell blocks using Leica Bond (Leica Biosystems, Buffalo Grove, IL, USA) and Ventana Benchmark Ultra (Ventana Medical Systems, Tucson, AZ, USA) immunostainers with satisfactory negative and positive controls. The antibodies, clones, dilutions, and manufacturers are summarized in Table 2. Expression of BCL6, CD10 and MUM1 in the lymphoma cells was evaluated with a cutoff of 30% for each antibody. The cell-of-origin (COO) of the selected cases was classified into either germinal center B-cell (GCB) or non-GCB subtype according to the Hans algorithm55. The cutoff for BCL2 and MYC expression was set at 50% and 40%, respectively, according to the literature56. Ki67 was utilized to assess the proliferation rate of lymphoma cells (0–100%). The immunostains of other antibodies were graded as negative or positive.
In situ hybridization
Digoxigenin-labeled probes (DAKO) were used to detect EBV-encoded small RNA-1 and -2 (EBER-1/2) for EBV infection, immunoglobulin kappa light chain (IGK), and immunoglobulin lambda light chain (IGL). The assays were performed on a Leica Bond platform with prediluted probes for EBER, IGK, and IGL (Leica Biosystems).
Fluorescence in situ hybridization (FISH)
FISH studies for BCL2, BCL6, and MYC rearrangements were performed on 5.0 μm unstained sections and prepared from formalin-fixed paraffin-embedded (FFPE) tissue blocks. Break-apart probes of BCL2, BCL6, and MYC (Vysis® Inc., Downers Grove, IL, USA) were used to interrogate the loci of interest. Prior to hybridization, slides were pretreated using an automated VP 2000™ processor (Abbott Molecular Inc., Abbott Park, IL, USA) with standard protocols. Following pretreatment, the cells and probes underwent co-denaturation at 73 °C for 5 min using the Hybrite™ hybridization system (Abbott Molecular Inc.) and then incubated overnight at 39 °C in a humidified chamber. Nuclei were counterstained with 4, 6-diamidino-2-phenylindole (DAPI) and the slides were analyzed using an Olympus BX53 fluorescence microscope (Olympus, Tokyo, Japan). Hybridization signals were assessed from 30 interphase nuclei with an established cut-off of 20% for rearrangement positivity.
Molecular assays for gene rearrangements
Polymerase chain reaction (PCR) assays were utilized to detect gene rearrangements of immunoglobulin heavy chain (IGH) and T-cell receptor gamma (TRG). Briefly, genomic DNA was extracted from FFPE tissues. Then, the gene rearrangement products for IGH and TRG were amplified by PCR and analyzed on an ABI 3500XL genetic analyzer (Applied Biosystems, Foster City, CA, USA) with appropriate positive and negative controls. For IGH gene rearrangements, two sets of primers were used. The primers were specific for the variable and joining regions of the IGH gene and the sequences were published previously57,58. For TRG gene rearrangements, three sets of primers specific for different gamma variable regions were paired with different joining region primers of the TRG gene59.
Statistical analyses
Univariate survival analysis was performed to identify potentially significant characteristics; all cases (including ours and those in the literature) with adequate clinical and survival data were included in the analyses. With the exception of stratifying overall survival (OS) by region where the study was conducted (Japan, West, and other East Asia), the study by Kaji et al. was excluded due to lack of adequate individual case data. The characteristics identified as significant or nearly significant by univariate analysis were included in the multivariable analysis.
Survival curves for each categorical variable were generated using the Kaplan-Meier method. Survival rates were compared using the log-rank test of equality across strata; these analyses were performed using GraphPad Prism software, version 9.2.0 (GraphPad Software, La Jolla, CA, USA). A Cox proportional hazard regression model was used for testing multiple variables and interactions between variables in Stata 16 (Stata Statistical Software: Release 16) (StataCorp LLC., College Station, TX, USA). The Pearson’s chi-squared test and the Fisher’s exact test were used to assess categorical variable characteristics among multiple groups. Significance level was set at 0.05.
Results
Clinical features
The demographics and clinical characteristics of the 55 cases of EB-LBCL in our study are summarized in Table 3. There was no gender predisposition, with a male to female ratio of approximately 1:1 (28:27). Most patients were elderly with a median age of 80 years (range 35–99 years). HIV and hepatitis C virus (HCV) infections were detected in 5% (1/20) and 10% (2/20) of patients, respectively. Seventy-nine percent (30/38) of patients had risk factors for fluid overload, including congestive heart failure, chronic renal failure, and cirrhosis, and 47% (15/32) of patients developed edema or benign effusions prior to or simultaneously with the malignant effusions.
The pleural cavity was the most common site of lymphomatous effusion (40/55, 73%), with exclusive pleural involvement in 34 (62%) cases (Table 3) (Fig. 1A, B). Involvement of the pericardial cavity was seen in 17 cases (31%), including 11 (20%) with exclusive pericardial involvement. Peritoneal effusions were noted in 4 (7%) cases. Overall, six cases (11%) had concurrent involvement of both pleural and pericardial cavities. Forty-eight percent (16/33) of cases had large effusions of at least one liter in volume. None of the 55 cases had solid mass formation of lymphoma in the body cavities or elsewhere prior to, concurrently, or during initial lymphoma staging. Additional information from a staging bone marrow biopsy was available in 20 cases and all had no involvement of large B-cell lymphoma, although two cases showed incidental monoclonal B-cell lymphocytosis (MBL).
Morphologic features and diagnoses
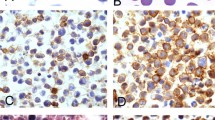
The malignant effusions in the body cavities mostly contained numerous lymphoma cells, which were large with variable cytologic features. The detailed cytologic characteristics were recorded in 27 cases. Eleven (41%) cases displayed cells with centroblastic cytology (Fig. 2A, B), eight (30%) revealed cells with pleomorphic or anaplastic cytology (Fig. 2C, D), and seven (26%) contained cells with immunoblastic cytology (Fig. 2E). One case (3%) had plasmablastic morphology (Fig. 2F), with the tumor cells expressing B-cell markers (CD19, CD20 and CD22) but not CD138.
A spectrum of cytomorphology is noted, including centroblastic cytology (A: Diff-Quick, 600× and B: H&E, 600×), large pleomorphic or anaplastic morphology (C: Wright-Giemsa, 600× and D: cell block, H&E, 400×), immunoblastic cytology (E: H&E, 600×), and plasmablastic morphology (F: Wright-Giemsa, 600×).
Nine cases with plasmablastic immunophenotype were excluded. We also excluded six cases with a history of low-grade B-cell lymphoma, six cases lacking HHV8 studies, and one case with a history of renal transplant.
Immunohistochemistry and in situ hybridization
The results of immunohistochemical stains and EBER-ISH studies are summarized in Table 4. EB-LBCL expressed CD45 (93%, 40/43), CD20 (98%, 53/54), and PAX5 (100%, 23/23) (Fig. 3A, C), whereas CD3 was negative in all 46 cases stained. CD138 was mostly negative (Fig. 3D) except one case with focal positivity (1/27, 4%). Three of 47 (6%) cases were positive for EBV, and HHV8 was negative in all 55 cases (Fig. 3E, F). BCL6, CD10 and MUM1 were positive in 68% (26/38), 15% (7/48), and 88% (28/32) of cases, respectively (Fig. 3G, I), and thus most cases (30/36, 83%) were classified as non-GCB subtype per the Hans algorithm. BCL2 and MYC were positive in 83% (30/36) and 43% (12/28) of cases, respectively; overall, 10 of 26 (38%) cases were considered dual-expressor lymphomas (DEL), with over-expression of both MYC and BCL2. CD30 was variably positive in 27% (8/30) of cases and cyclin D1 was negative in all 23 cases stained. Ki67 proliferation indices were available in 35 cases, with a median proliferation rate of 80% (range 30–95%).
Molecular and genetic studies
Rearrangements of BCL2, BCL6, and MYC genes were detected in 2/22 (9%), 7/20 (35%), and 6/29 (21%) cases, respectively (Table 4). Three of 25 (12%) cases had both MYC and BCL2 and/or BCL6 rearrangements and were therefore classified as “high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements”. By PCR assays, three of four (75%) cases were positive for clonal IGH gene rearrangement, whereas TRG gene rearrangement was not detected in the one case tested.
Clinical course, treatment, and prognosis
Clinical management information was available in 34 cases (Table 3). Fifteen (44%) patients were treated with drainage of the body fluids alone without any chemotherapy, four (12%) received rituximab alone during the course of the disease, and fifteen (44%) received chemotherapy, mostly with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like regimens, including R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; 10 cases) and EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and hydroxydaunorubicin; 3 cases).
A total of 50 cases had sufficient clinical follow-up data, with a median follow-up interval of three months (range 1–180 months). Seven of 48 (15%) patients developed solid lymphoma masses during follow-up, with a median interval of 41 months (range 5–180 months). Of those who were treated with chemotherapy, 14 of 22 (64%) patients achieved complete remission (CR) initially, seven (32%) patients showed disease persistence or progression, and one (4%) patient had partial response. Of the 15 patients treated with drainage only, six patients were discharged to hospice care without chemotherapy, either due to other severe comorbidities or refractory disease; the remaining nine were managed with drainage alone, and four of five patients achieved CR, with one patient remaining in CR and alive 82 months after initial diagnosis. Eventually, 22 of 39 (56%) patients died. Overall, EB-LBCL in our study had an unfavorable prognosis, with a median OS of 14.9 months and a 2-year OS of 44%.
Literature review of EB-LBCL
Clinical features
A total of 202 cases of EB-LBCL were included in this review, including 55 from our study and 147 from the literature. The clinicopathologic features and statistical results are listed in Tables 3–5. Among all cases of EB-LBCL (those in our study and in the literature), most occurred in elderly patients (median age 79 years, excluding the Kaji group due to lack of detailed individual data) with a male to female ratio of approximately 5:4 (113:89) and a risk of fluid overload in 56% (89/158) of patients. None of these patients had solid mass formation of lymphoma prior to or concurrent with the diagnosis of EB-LBCL. The patients in our study had a significantly higher risk of fluid overload (30/38, 79%), compared to the Japanese group (41/82, 50%, p = 0.003) and the non-Japanese group (18/38, 47%, p = 0.008). The Japanese group had a significantly higher incidence of pericardial effusion (49/87, 56%, including mixed effusions) compared to our group (17/55, 31%, p = 0.004) and the all-non-Japanese group (28/115, 24%, p < 0.0001) (Tables 3, 5, and Fig. 5A). In addition, the Japanese group tended to present with mixed effusions, particularly simultaneous pleural and pericardial effusions (Tables 3, 5, and Fig. 5A).
Most (85%, 71/84) of the patients in the Japanese group received chemotherapy, compared to 46% (42/91) in the all-non-Japanese group (p < 0.0001) and 56% (19/34) in our group (p = 0.002). The rates of complete response to chemotherapy and/or drainage were not significantly different among these three groups (Tables 3 and 5).
Pathological features
The results of immunohistochemical stains, EBER-ISH, and molecular studies are summarized in Table 4. Most cases stained positively for CD45 (76%, 55/72) and CD20 (96%, 182/190). Sixteen of 171 (9%) cases were positive for EBV, and HHV8 was not detected in any of the 202 cases. BCL6, CD10 and MUM1 were positive in 51% (62/121), 16% (24/152), and 75% (84/112) of cases, respectively. Overall, the non-GCB subtype accounted for 79% (98/124) cases of EB-LBCL. Expression of BCL2 and MYC was detected in 73% (70/96) and 34% (21/61) of cases, respectively, and 30% (19/63) of cases were classified as DEL. Rearrangements of BCL2, BCL6, and MYC genes were detected in 11% (6/54), 29% (16/55), and 19% (20/107), respectively. Six percent (6/100) of cases had both MYC and BCL2 and/or BCL6 rearrangements. Taken together, among the three groups (our study, Japanese, and non-Japanese), there were no significant differences regarding immunophenotype, EBV status, COO subtype, and rearrangements of BCL2, BCL6 and MYC genes (Tables 4 and 5).
Survival analyses
Our survival analyses demonstrated that the patients in the Kaji group had significantly better OS than all other groups, including our cohort (Fig. 4A). In addition, the non-Kaji group tended to have better OS than the other East Asian group, the Western group, and our cohort, although these observations were not statistically significant (Fig. 4A).
A The Kaji group had significantly better OS than the non-Kaji group, our group, and the other East Asian and Western groups from the literature. B The Kaji and non-Kaji groups were combined to form the Japanese group, and the Western and Other East Asian groups in the literature were combined to form the non-Japanese group; the Japanese group had significantly better OS than the non-Japanese group and our study.
Since the Kaji and non-Kaji groups had the best OS and were both comprised of cases from Japanese studies, the difference in OS among Japanese and non-Japanese cases was further investigated. We therefore combined the Kaji and non-Kaji groups to form the “Japanese group” (Fig. 4B). In addition, the other East Asian and Western groups from the literature showed no difference in OS (Fig. 4A), and they were combined and referred to as the “non-Japanese group” (Fig. 4B). Overall, the OS of the Japanese group (median 63.6 months) was significantly better than that of the non-Japanese group (median 11.0 months, p = 0.0002) and of our study (median 14.9 months, p < 0.0001) (Fig. 4B). There was no significant difference in OS between our group and the non-Japanese group (p = 0.6846) (Fig. 4B).
The presence of pericardial effusion, either alone or mixed, was associated with improved OS when compared to the presence of pleural or peritoneal effusion (Fig. 5B); however, only the comparison of OS among those with pericardial effusion, either alone or mixed, (“pericardial total”) and those with peritoneal effusion, either alone or mixed, (“peritoneal total”) reached statistical significance (p = 0.0063) (Fig. 5B). In addition, patients that received chemotherapy had significantly improved OS compared to those that did not receive chemotherapy (p = 0.0016) (Fig. 5C).
A Effusion sites of our cohort, the Japanese group, and the non-Japanese group in the literature. The Japanese group tended to present with more pericardial and mixed effusions than the other groups. B Statistical analyses of the cases in our study and the literature showed that pericardial effusion, either alone or mixed, was associated with better OS than pleural or peritoneal effusions; however, only the comparison between pericardial total and peritoneal total was statistically significant. C Chemotherapy improved the OS significantly.
Dual-expressor status, COO subtype status, and the presence of EBV infection provided no significant prognostication to the OS of EB-LBCL (Fig. 6A–C). Unsurprisingly, MYC rearrangement was associated with a significantly unfavorable OS (p = 0.0054) (Fig. 6D), and all 10 patients with MYC rearrangement died within one year.
The predictors included in the multivariable analysis were as follows: region where study was conducted (Japan vs. non-Japan), age group using median age as cutoff (<79 or ≥79 years), and the presence of peritoneal effusion. No significant interactions between these variables were identified. MYC rearrangement status was excluded from the model due to small sample size. Japanese residence was an independent favorable prognostic factor (HR = 0.475; 95% CI, 0.216–1.044, p = 0.064), while the presence of peritoneal effusion (HR = 3.652; 95% CI, 1.763–7.565, p = 0.004), and age of at least 79 years (HR = 2.251; 95% CI, 1.287–3.938, p < 0.001) were independent unfavorable prognostic factors.
Discussion
Etiology of EB-LBCL
Although it is difficult to accurately determine the prevalence of EB-LBCL due to its rarity, EB-LBCL is likely more common than PEL. According to the 5-year data in Miyagi Prefecture, Japan, the incidence of EB-LBCL is 0.1% among newly diagnosed lymphoid leukemias and/or lymphomas60. In a recent Japanese study, the incidence of EB-LBCL was >12 times higher than that of PEL5. In addition, based on the limited data from a single institution in our study, there were 24 EB-LBCLs and 11 PELs within the last 20 years. The lower EB-LBCL to PEL ratio in our observation is likely due to the higher prevalence of HHV8+ patients in the US than in Japan61.
Currently, the etiology of EB-LBCL remains uncertain. Fluid retention has been considered to be one of the potential pathogeneses of EB-LBCL44. According to the overall findings from our study and the literature, 56% (89/158) of patients had underlying conditions placing them at risk for fluid retention, including congestive heart failure, cirrhosis, and chronic renal failure.
The association of HCV with EB-LBCL has been actively explored, albeit with inconsistent findings. HCV infection was reported in up to 22–33% cases of EB-LBCL7,40,44,62, whereas in a recent large Japanese series only one of 61 cases was positive5. The rates of HCV infection in our study (10%) and in the non-Japanese group from the literature (15%) are higher than those in the Japanese group (6%). Based on our review, 16 of 138 (12%) cases had peritoneal involvement; among the 10 cases that were HCV+, six cases (60%) had peritoneal involvement. In other words, six of the 16 (38%) cases with peritoneal involvement were HCV+. Therefore, a substantial proportion of the peritoneal cases were likely associated with cirrhosis caused by HCV. HCV has been shown to be lymphotropic and clonal expansions of B-cells have been detected in HCV-infected patients. Moreover, the detection of HCV-RNA in peritoneal fluid indicates that persistent antigenic stimulation may play a causative role in EB-LBCL by promoting clonal expansion of intraperitoneal B-cells63. Besides EB-LBCL, HCV has been implicated in lymphomagenesis for several other non-Hodgkin lymphoma subtypes64,65. Studies have shown that HCV-associated lymphomas may arise from B cells that were activated by the HCV-E2 protein, and the putative E2 receptor (CD81) forms a signaling complex including CD1966. Other studies have proposed that the lymphomagenesis of HCV may be partially through stimulating BCL2 over-expression and MYC amplification67,68.
EBV infection has been detected in DLBCL, with a prevalence of 5–15% among Asian and Latin American patients and <5% among Western patients69. According to our data, 12% (23/193) of EB-LBCL cases were EBV+, which is similar to the incidence of EBV+ DLBCL. Nevertheless, the overall findings suggest that EBV infection may contribute to a small percentage of EB-LBCL cases.
Immunosuppression is likely not a major predisposing factor for EB-LBCL, although rare cases have been reported in patients with congenital immunodeficiency, including common variable immunodeficiency (CVID)10,13. In our study and in the literature, only three of 125 (2%) patients had HIV infection (six cases with organ transplantation were excluded). However, EB-LBCL tends to occur in elderly patients, with a median age of 79 years based on our review; therefore, immunosenescence or altered immunosurveillance of the elderly might contribute to the development of EB-LBCL.
Interestingly, the non-GCB subtype accounts for the majority (79%) of EB-LBCL. It is likely that the lymphoma cells in the effusions tend to demonstrate terminal differentiation to the plasmablastic or plasmacytic stage, in contrast to the conventional solid DLBCL.
Based on our study and literature review, MYC, BCL2, and BCL6 gene rearrangements were detected in 19%, 11%, and 29% of EB-LBCL cases, respectively. In contrast, these rearrangements are less often identified in PEL, supporting different pathogeneses for these two entities. The genetic alteration of MYC has been proposed to be involved in the pathogenesis of EB-LBCL70. MYC rearrangement is more common in EB-LBCL (19%) than in conventional DLBCL (~10%)71, and this rearrangement is associated with a poor prognosis in both entities. One case of EB-LBCL was reported to have t(9;14)(p13;q32), involving PAX5 and IGH24.
EB-LBCL has been reported to display complex genomic changes19,70, including complex numeric and structural chromosomal abnormalities. A recent study with next generation-sequencing assays on 11 cases of EB-LBCL revealed a complex genomic landscape with frequent mutations, copy number aberrations, and translocations. In addition to the high frequency of somatic hypermutations, the most common recurrent mutations involved HIST1H1E and MYD8853. These aberrations are typically found in the activated B-cell subtype (ABC) of DLBCL and enriched in extranodal DLBCL. Other common mutations were detected in BTG1 and/or BTG2, IRF4 and SYNE153. Moreover, biallelic inactivation of PRDM1 due to deletions and/or mutations was noted in a subset of EB-LBCL53.
Differential diagnoses of EB-LBCL
EB-LBCL may be confused with other cavity-based effusion or solid B-cell lymphomas, particularly PEL and pyothorax-associated lymphoma (PAL) (Table 6). PEL is a rare entity of large B-cell lymphoma as defined by the 2017 WHO Classification. It mostly occurs in young to middle-aged HIV+ males and occasionally in patients with other immunodeficient conditions, including solid organ transplantation and immunosenescence in the elderly. PEL commonly exhibits plasmablastic cytology and is typically negative for pan-B-cell markers (CD20, CD79a, and PAX5). The tumor cells are mostly positive for CD45, CD30 and CD138. Characteristically, HHV8 is consistently positive by definition, while EBV infection is noted in most cases. In contrast to PEL, EB-LBCL occurs in elderly patients without HIV infection. Moreover, EB-LBCL usually expresses pan-B-cell markers with no HHV8 infection and has a low association with EBV. Therefore, EB-LBCL and PEL appear to be biologically distinct entities.
PAL is a prototype of DLBCL associated with chronic inflammation in the thorax72. However, compared to EB-LBCL, patients with PAL are much younger and usually have a long history of chronic pyothorax or chronic pleuritis due to therapeutic artificial pneumothorax or tuberculous pleuritis. PAL presents with mass lesions in the pleura and/or lung near the pleura and is strongly associated with EBV infection.
It is necessary to separate EB-LBCL from conventional solid DLBCL with concurrent or subsequent malignant effusions (more discussion below). In addition, other high-grade B-cell or T-cell lymphomas occasionally manifest as lymphomatous effusions, including Burkitt lymphoma, blastoid/pleomorphic mantle cell lymphoma, peripheral T-cell lymphoma, and anaplastic large-cell lymphoma, which can be separated from EB-LBCL based on the clinical presentations and immunophenotypic features.
Clinical management and survival of EB-LBCL
Due to the rarity of EB-LBCL, no standard therapeutic regimen has been recommended thus far. Based on findings from the literature and our study, the OS was highly variable; some patients died within a short period of follow-up, while some had complete remission with drainage alone and no chemotherapy12,18,19. Therefore, select patients with EB-LBCL may benefit from a watchful-waiting approach after drainage without systemic chemotherapy; however, specific selection criteria haven’t been established for this group of patients. A recent review summarized 13 cases of EB-LBCL who survived longer than one year without treatment; in contrast to other more aggressive EB-LBCLs, these cases tended to occur in older patients with fewer comorbidities, a lower rate of viral infections (such as HCV), and fewer aggressive cytomorphological features21. Nevertheless, the mechanism underlying the complete remission of EB-LBCL with drainage alone remains mysterious.
In the Japanese study from Kaji et al., 56 patients with EB-LBCL received immediate systemic chemotherapy, and 48 (86%) patients were treated with first-line therapy including CHOP or a CHOP-like regimen with or without rituximab5. The overall response rate was 95%, and 73% achieved complete remission. The 2-year OS and progression free survival rates were 84.7% and 73.8%, respectively, indicating a favorable prognosis. These response and survival rates were higher than those observed for similarly aged patients with nodal DLBCL5.
To our knowledge, no clinical trials have been conducted on patients with EB-LBCL. This is likely due to the limited number of cases reported in the literature, and it may also reflect a lack of consensus regarding whether or not EB-LBCL represents a distinct entity. Based on the data from our study and the literature, systemic chemotherapy with CHOP or a CHOP-like regimen should be recommended for qualified patients without significant contraindications or comorbidities. Chemotherapy with R-CHOP following drainage of the effusion is likely beneficial in select patients with prolonged survival. Drainage of effusions alone may be considered for patients who cannot tolerate chemotherapy or elderly patients with significant comorbidities who may not benefit from chemotherapy.
One review suggested that Japanese ethnicity, pericardial effusion, and CD20 expression were favorable prognostic factors, whereas peritoneal effusion and CD20 negativity were unfavorable prognostic factors54. History of fluid overload, HCV, and peritoneal effusion were significantly correlated with patients younger than 70 years, while pericardial and pleural effusions were significantly correlated with patients aged 70 years and above. In the multivariate analysis, age over 70 years and CD20 expression were independent favorable prognostic factors, and EB-LBCL with positive CD20 expression had a better outcome after rituximab treatment54,62. Therefore, chemotherapy with rituximab may be an appropriate standard therapy for EB-LBCL with CD20 expression. Overall, these findings reported in the literature are mostly in line with the findings from our study. Our study further supports the idea that non-Japanese residence and presence of peritoneal effusion are independently associated with an unfavorable prognosis. It is not entirely clear as to why the presence of peritoneal effusion is an independent poor prognostic factor. One possible explanation is that peritoneal effusions tend to be associated with cirrhosis, a disease that carries high morbidity and mortality on its own. It is also uncertain as to why pericardial effusion, either alone or concurrent, is associated with significantly improved OS. It is possible that pericardial effusions tend to cause the most symptoms early on, which leads to earlier detection of lymphoma. We did not include CD20-negative/CD138+ EB-LBCLs in our study since most of them are of plasmablastic immunophenotype; these cases will be studied separately.
Should EB-LBCL be considered a distinct entity?
It was uncertain whether or not EB-LBCL should be considered as part of the spectrum or a continuum of conventional DLBCL, not otherwise specified. Based on our findings and literature review, EB-LBCL primarily occurs in the elderly with a median age of 79 years, in contrast to the mid-60 s of conventional DLBCL. In addition, according to the Hans algorithm, the non-GCB subtype accounts for 79% of EB-LBCL, which is significantly higher than that of conventional DLBCL.
In our study, only seven of 48 EB-LBCLs relapsed as solid masses, with a median interval of 41 months (range 5–180 months). Moreover, in another ongoing study, we have collected a large number of effusion DLBCLs in the body cavities with antecedent and/or concurrent solid forms (so called solid-effusion DLBCLs). We have noticed significant differences between EB-LBCL and solid-effusion DLBCL regarding demographic characteristics, immunophenotypic features, molecular genetic changes, clinical behaviors, and outcomes (data not published). Therefore, EB-LBCL likely represents a distinct entity rather than a continuum of solid lymphoma.
Currently, consensus criteria for diagnosis and classification of EB-LBCL have not yet been created. Since 1997, several classification systems have been proposed to characterize and classify EB-LBCL, with emphases on clinical features, morphology, immunophenotype, HHV8 status, and molecular genetic aberrations (particularly MYC gene status). Moreover, there is a lack of standardized nomenclature for this entity with many different terminologies used. Particularly, the different diagnostic terms, as designated in many case reports and series, can be confusing. Therefore, there is a need to standardize the diagnostic criteria and nomenclature of EB-LBCL, in order to maintain international uniformity and promote effective communication among pathologists and other clinical teams. Furthermore, it may be worthwhile to consider EB-LBCL as a disease entity separate from conventional DLBCL for the reasons discussed above.
In the 2017 WHO Classification of Lymphoid Neoplasms, PEL is defined as a large B-cell neoplasm that typically occurs in the setting of immunodeficiency and lacks pan-B-cell markers; in this classification, PEL is universally associated with HHV82. Now that there is increased awareness of an effusion-based large B-cell lymphoma that is not associated with HHV8, a unified term for this type of lymphoma is needed. EB-LBCL and PEL are likely unrelated entities with different etiology, immunophenotype, and prognosis. On the other hand, their clinical presentations (lymphomas manifesting as malignant effusions without an associated solid component) are nearly the same. Therefore, as in the classification of anaplastic lymphoma kinase (ALK)-positive and ALK-negative anaplastic large cell lymphomas (ALCLs), it may be worthwhile to consider EB-LBCL as “HHV8-negative PEL”, similar to that proposed by some publications8,9,10,12,17. Nevertheless, an ultimate term for EB-LBCL should be determined based on consensus among the hematopathology and hematology societies.
In conclusion, compared to conventional DLBCL, EB-LBCL demonstrates unique clinicopathologic features; it tends to occur in elderly, otherwise immunocompetent individuals with no antecedent or concurrent solid lymphomas. There is no HHV8 infection and EBV is mostly negative. It is typically of non-GCB subtype and is associated with a worse prognosis than previously reported, although select patients may be managed conservatively. Taken together, our data suggest that EB-LBCL is a unique entity that necessitates consensus criteria. In addition, consensus terminology is needed to reflect the nature of this entity and to replace many different terms used in the literature.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Cesarman, E. et al. Kaposi’s sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am. J. Pathol. 149, 53–7 (1996).
Swerdlow, S. H. et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood 127, 2375–90 (2016).
Carbone, A. et al. Kaposi’s sarcoma-associated herpesvirus DNA sequences in AIDS-related and AIDS-unrelated lymphomatous effusions. Br. J. Haematol. 94, 533–43 (1996).
Chen, B. J. et al. Primary effusion lymphoma in taiwan shows two distinctive clinicopathological subtypes with rare human immunodeficiency virus association. Histopathology 72, 930–44 (2018).
Kaji, D. et al. Primary human herpesvirus 8-negative effusion-based lymphoma: a large B-cell lymphoma with favorable prognosis. Blood Adv. 4, 4442–50 (2020).
Usmani, A., Walts, A. E., Patel, S., Alkan, S. & Kitahara, S. HHV8-negative effusion based lymphoma: a series of 17 cases at a single institution. J. Am. Soc. Cytopathol. 4, 37–43 (2015).
Wu, W., Youm, W., Rezk, S. A. & Zhao, X. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma: Report of a rare case and review of 54 cases in the literature. Am. J. Clin. Pathol. 140, 258–73 (2013).
Inoue, Y., Tsukasaki, K., Nagai, K., Soda, H. & Tomonaga, M. Durable remission by sobuzoxane in an HIV-seronegative patient with human herpesvirus 8-negative primary effusion lymphoma. Int. J. Hematol. 79, 271–5 (2004).
Nonami, A. et al. Human herpes virus 8-negative primary effusion lymphoma (PEL) in a patient after repeated chylous ascites and chylothorax. Intern. Med. 43, 236–42 (2004).
Hisamoto, A. et al. Human herpes virus-8-negative primary effusion lymphoma in a patient with common variable immunodeficiency. Leuk. Lymphoma 44, 2019–22 (2003).
Matsumoto, Y. et al. Human herpesvirus 8-negative malignant effusion lymphoma: a distinct clinical entity and successful treatment with rituximab. Leuk. Lymphoma 46, 415–9 (2005).
Yamaoka, O. et al. Indolent primary effusion lymphoma-like lymphoma in the pericardium: a case report and review of the literature. J. Cardiol. Cases 19, 148–52 (2019).
Lam, G. K. et al. Primary effusion lymphoma (PEL)-like lymphoma in a child with congenital immunodeficiency. Pediatr Blood Cancer 63, 1674–6 (2016).
Kim, H. J., Lee, K., Yoon, C. H. & Bang, S. M. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma presenting with cardiac tamponade: a case report. Medicine 96, e8010 (2017).
Nakamura, H., Tsuta, K., Nakagawa, T., Hirai, R. & Ota, Y. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma in the pericardium: a case with latency type III epstein-barr virus infection showing good prognosis without chemotherapy. Pathol. Res. Pr. 211, 1010–3 (2015).
Kashiwagi, T. et al. HIV-negative, HHV-8-unrelated primary effusion lymphoma-like lymphoma with genotypic infidelity and c-MYC expression. Ann. Hematol. 93, 1609–10 (2014).
Chen, B. J. & Chuang, S. S. Lymphoid neoplasms with plasmablastic differentiation: a comprehensive review and diagnostic approaches. Adv. Anat. Pathol. 27, 61–74 (2020).
Nakatsuka, S. et al. Self-limited effusion large B-cell lymphoma: two cases of effusion lymphoma maintaining remission after drainage alone. Acta Haematol. 130, 217–21 (2013).
Terasaki, Y. et al. Disappearance of malignant cells by effusion drainage alone in two patients with HHV-8-unrelated HIV-negative primary effusion lymphoma-like lymphoma. Int. J. Hematol. 94, 279–84 (2011).
Saiki, M. et al. Human herpesvirus-8 negative primary effusion lymphoma with complete clinical remission after removal of ascites. Rinsho Ketsueki 43, 548–53 (2002).
Kim, M. et al. Human herpesvirus 8-negative effusion-based lymphoma with indolent clinical behavior in an elderly patient: a case report and literature review. Oncol. Lett. 20, 343 (2020).
Tsai, M. C., Kuo, C. C., Su, Y. Z., Hsieh, Y. C. & Chuang, S. S. Effusion-based lymphoma with morphological regression but with clonal genetic features after aspiration. Diagn. Cytopathol. 46, 685–9 (2018).
Hermine, O., Michel, M., Buzyn-Veil, A. & Gessain, A. Body-cavity-based lymphoma in an HIV-seronegative patient without kaposi’s sarcoma-associated herpesvirus-like DNA sequences. N. Engl. J. Med. 334, 272–3 (1996).
Ichinohasama, R. et al. Herpes virus type 8-negative primary effusion lymphoma associated with PAX-5 gene rearrangement and hepatitis C virus: a case report and review of the literature. Am. J. Surg. Pathol. 22, 1528–37 (1998).
Rodriguez, J., Romaguera, J. E., Katz, R. L., Said, J. & Cabanillas, F. Primary effusion lymphoma in an HIV-negative patient with no serologic evidence of kaposi’s sarcoma virus. Leuk. Lymphoma 41, 185–9 (2001).
Ashihara, E. et al. Human herpes virus 8-negative primary effusion lymphoma in a patient with a ventriculoperitoneal shunt tube. Int. J. Hematol. 74, 327–32 (2001).
Nakamura, Y. et al. Primary effusion lymphoma of the left scrotum. Intern. Med. 42, 351–3 (2003).
Shimazaki, M. et al. An unusual case of primary effusion lymphoma in a HIV-negative patient not pathogenetically associated with HHV8. Eur. J. Haematol. 71, 62–7 (2003).
Paner, G. P., Jensen, J., Foreman, K. E. & Reyes, C. V. HIV and HHV-8 negative primary effusion lymphoma in a patient with hepatitis C virus-related liver cirrhosis. Leuk. Lymphoma 44, 1811–4 (2003).
Takao, T. et al. Rituximab is effective for human herpesvirus-8-negative primary effusion lymphoma with CD20 phenotype associated hepatitis C virus-related liver cirrhosis. Am. J. Hematol. 77, 419–20 (2004).
Fujiwara, T. et al. Primary effusion lymphoma of the pericardial cavity carrying t(1;22)(q21;q11) and t(14;17)(q32;q23). Cancer Genet Cytogenet 156, 49–53 (2005).
Fujisawa, S., Tanioka, F., Matsuoka, T. & Ozawa, T. CD5+ diffuse large B-cell lymphoma with c-myc/IgH rearrangement presenting as primary effusion lymphoma. Int. J. Hematol. 81, 315–8 (2005).
Youngster, I., Vaisben, E., Cohen, H. & Nassar, F. An unusual cause of pleural effusion. Age Ageing 35, 94–6 (2006).
Taira, T. et al. Establishment of a human herpes virus-8-negative malignant effusion lymphoma cell line (STR-428) carrying concurrent translocations of BCL2 and c-MYC genes. Leuk. Res. 31, 1285–92 (2007).
Nemr, S., Mayor-Modesto, M. H., Schwartz, S. & Summerhill, E. M. A 92-year-old woman with recurrent pleural effusions. Chest 134, 196–9 (2008).
Tsagarakis, N. J. et al. Report of an HIV and HHV-8 negative case of primary effusion lymphoma with idiopathic T4 lymphocytopenia. Int. J. Hematol. 90, 94–8 (2009).
Takahashi, T. et al. HIV-negative, HHV-8-unrelated primary effusion lymphoma-like lymphoma: report of two cases. Am. J. Hematol. 85, 85–7 (2010).
Wang, T., Nava, V. E., chter, G. P., Lichy, J. H. & Liu, M. L. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: a patient successfully treated with pleurodesis. J. Clin. Oncol. 29, e747–50 (2011).
Kim, K. H. et al. A case of human herpes virus-8 unrelated primary effusion lymphoma-like lymphoma presented as pleural effusion. Tuberc. Respir. Dis. 73, 336–41 (2012).
Saini, N. et al. HHV8-negative primary effusion lymphoma of B-cell lineage: two cases and a comprehensive review of the literature. Case Rep. Oncol. Med. 2013, 292301 (2013).
Xiao, J., Selvaggi, S. M., Leith, C. P., Fitzgerald, S. A. & Stewart, J. 3rd Kaposi sarcoma herpesvirus/human herpesvirus-8-negative effusion-based lymphoma: report of 3 cases and review of the literature. Cancer Cytopathol. 121, 661–9 (2013).
Kumode, T. et al. Clinical importance of human herpes virus-8 and human immunodeficiency virus infection in primary effusion lymphoma. Leuk. Lymphoma 54, 1947–52 (2013).
Mohammad, F. et al. A unique case of malignant pleuropericardial effusion: HHV-8-unrelated PEL-like lymphoma-A case report and review of the literature. Case Rep. Oncol. Med. 2014, 436821 (2014).
Alexanian, S., Said, J., Lones, M. & Pullarkat, S. T. KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am. J. Surg. Pathol. 37, 241–9 (2013).
Nussinson, E. et al. Primary effusion lymphoma-like lymphoma in a patient with inflammatory bowel disease. World J. Gastroenterol. 20, 857–62 (2014).
Fan, H. B. et al. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma in a patient with hepatitis B virus-related liver cirrhosis: a case report. J. Res. Med. Sci. 19, 190–2 (2014).
Dai, H., Cherian, R. & Mathur, S. Primary body cavity-based large B-cell lymphoma in an HIV and HHV-8 negative, HCV positive patient: a case report and literature review. Lab. Med. 45, 136–40 (2014).
Choi, J. W., Kim, Y., Lee, J. H. & Kim, Y. S. Human herpesvirus 8-negative and epstein-barr virus-positive effusion-based lymphoma in a patient with human immunodeficiency virus. J. Pathol. Transl. Med. 49, 409–12 (2015).
Zhang, E. et al. HHV-8-unrelated primary effusion-like lymphoma associated with clonal loss of inherited chromosomally-integrated human herpesvirus-6A from the telomere of chromosome 19q. Sci. Rep. 62, 2730 (2016).
Oki, M. et al. Primary effusion lymphoma-like lymphoma in a patient with neurofibromatosis type 1. Tokai J. Exp. Clin. Med. 41, 123–9 (2016).
Koeda, C. et al. A unique case of primary effusion lymphoma-like lymphoma showing disappearance and recurrence of the body cavity effusion. J. Gen. Fam. Med. 18, 38–41 (2017).
Kojima, M. et al. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma following tyrosine kinase inhibitor treatment for chronic myelogenous leukemia. J. Clin. Exp. Hematop. 57, 69–73 (2017).
Mendeville, M. et al. Aggressive genomic features in clinically indolent primary HHV8-negative effusion-based lymphoma. Blood. 133, 377–80 (2019).
Kubota, T. et al. Age and CD20 expression are significant prognostic factors in human herpes virus-8-negative effusion-based lymphoma. Am. J. Surg. Pathol. 42, 1607–16 (2018).
Hans, C. P. et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103, 275–82 (2004).
Johnson, N. A. et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 30, 3452–9 (2012).
Ramasamy, I., Brisco, M. & Morley, A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J. Clin. Pathol. 45, 770–5 (1992).
Slack, D. N., McCarthy, K. P., Wiedemann, L. M. & Sloane, J. P. Evaluation of sensitivity, specificity, and reproducibility of an optimized method for detecting clonal rearrangements of immunoglobulin and T-cell receptor genes in formalin-fixed, paraffin-embedded sections. Diagn. Mol. Pathol. 2, 223–32 (1993).
Shadrach, B. & Warshawsky, I. A comparison of multiplex and monoplex T-cell receptor gamma PCR. Diagn. Mol. Pathol. 13, 127–34 (2004).
Katsushima, H. et al. Non-biased and complete case registration of lymphoid leukemia and lymphoma for five years: A first representative index of japan from an epidemiologically stable miyagi prefecture. Leuk. Lymphoma 58, 80–8 (2017).
Chatlynne, L. G. & Ablashi, D. V. Seroepidemiology of kaposi’s sarcoma-associated herpesvirus (KSHV). Semin. Cancer Biol. 9, 175–85 (1999).
Kobayashi, Y. et al. Comparison of human herpes virus 8 related primary effusion lymphoma with human herpes virus 8 unrelated primary effusion lymphoma-like lymphoma on the basis of HIV: Report of 2 cases and review of 212 cases in the literature. Acta Haematol. 117, 132–44 (2007).
Ascoli, V. et al. Primary effusion burkitt’s lymphoma with t(8;22) in a patient with hepatitis C virus-related cirrhosis. Hum. Pathol. 28, 101–4 (1997).
Franzin, F. et al. Clonal B-cell expansions in peripheral blood of HCV-infected patients. Br. J. Haematol. 90, 548–52 (1995).
Gasparotto, D., De, Re. V. & Boiocchi, M. Hepatitis C virus, B-cell proliferation and lymphomas. Leuk. Lymphoma 43, 747–51 (2002).
Quinn, E. R. et al. The B-cell receptor of a hepatitis C virus (HCV)-associated non-hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood 98, 3745–9 (2001).
Ray, R. B., Meyer, K. & Ray, R. Suppression of apoptotic cell death by hepatitis C virus core protein. Virology 226, 176–82 (1996).
Fanidi, A., Harrington, E. A. & Evan, G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature 359, 554–6 (1992).
Castillo, J. J. et al. EBV-positive diffuse large B-cell lymphoma of the elderly: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 91, 529–37 (2016).
Ohshima, K. et al. Chromosomal and comparative genomic analyses of HHV-8-negative primary effusion lymphoma in five HIV-negative japanese patients. Leuk. Lymphoma 43, 595–601 (2002).
Rosenwald, A. et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large B-cell lymphoma: A study by the lunenburg lymphoma biomarker consortium. J. Clin. Oncol. 37, 3359–68 (2019).
Petitjean, B. et al. Pyothorax-associated lymphoma: a peculiar clinicopathologic entity derived from B cells at late stage of differentiation and with occasional aberrant dual B- and T-cell phenotype. Am. J. Surg. Pathol. 26, 724–32 (2002).
Pan, Z. G. et al. Extracavitary KSHV-associated large B-cell lymphoma: a distinct entity or a subtype of primary effusion lymphoma? study of 9 cases and review of an additional 43 cases. Am. J. Surg. Pathol. 36, 1129–40 (2012).
Acknowledgements
The authors thank Richard Bouffard, Susan Bell, Pei Hui, Lorraine Savoca, Cynthia DeRiso, and Mary Helie (Department of Pathology, Yale University School of Medicine) for their technical insight and assistance. The authors also thank Dr. Koji Izutsu (Department of Hematology, National Cancer Center Hospital, Tokyo, Japan) for providing additional survival data for our review.
Funding
This work was partially supported by the NIH/NCI grant 1R01CA233490-01A1.
Author information
Authors and Affiliations
Contributions
Z.P. and S.D.G. performed study concept and design, acquisition of data, analysis and interpretation of data, and preparation, review, and revision of the paper; J.Y. and M.L.X. provided study concept and design, acquisition of data, and review and revision of the paper; R.C.B., D.L.V.M., X.C., X.W., J.M., M.C., Y.X., L.E.B., P.L., Y.Z., E.D.H., E.W., Q.Z., and K.H.Y. provided acquisition and interpretation of data, and review and revision of the paper; S.D.G., H.T., A.M., R.G.H., and N.P. provided statistical analyses, and review and revision of the paper. T.S. provided advice on clinical data analyses. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
E.D.H. has received sponsored research from Eli-Lilly and served in the advisory board (Honoria) for Cytomx and Astellas. M.C. has served as a consultant for ALAB. M.L.X. has served in the Seattle Genetics lymphoma advisory board and as a consultant for Pure Marrow and Blueprint Medicines. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gisriel, S.D., Yuan, J., Braunberger, R.C. et al. Human herpesvirus 8-negative effusion-based large B-cell lymphoma: a distinct entity with unique clinicopathologic characteristics. Mod Pathol 35, 1411–1422 (2022). https://doi.org/10.1038/s41379-022-01091-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01091-x
This article is cited by
-
A rare case of fluid overload-associated large B-cell lymphoma and antigen loss at relapse
Journal of Hematopathology (2023)
-
Cavity-based lymphomas: challenges and novel concepts. A report of the 2022 EA4HP/SH lymphoma workshop
Virchows Archiv (2023)
-
Diffuse large B-cell lymphomas, not otherwise specified, and emerging entities
Virchows Archiv (2023)