Abstract
CIC-rearranged sarcoma is characterized by round cell undifferentiated histology, frequent expression of ETV4 and WT1, and aggressive behavior. A clinical encounter of a case with CIC-DUX4 fusion and ERG/CD31 co-expression prompted us to systematically investigate ERG and CD31 expression status in 30 archival cases of CIC-rearranged sarcoma. Half (15) of them showed moderate or strong ERG expression in <5–100% of tumor cells, among which nine showed heterogeneous membranous CD31 reactivity, including four cases each showing diffuse or strong expression. None of them showed uniformly strong and diffuse ERG/CD31 co-expression; however, three cases were initially interpreted and treated as angiosarcoma without response. Except for smaller superficial tumor enrichment, the clinicopathological characteristics of these nine cases of ERG+/CD31+ CIC-rearranged sarcoma did not differ from those of remaining 21 cases. Five showed focal hemorrhagic clefts/cysts, mimicking vascular spaces. All tumors expressed ETV4 and/or nuclear WT1, and fusion to DUX4 was confirmed in seven cases. Four tumors examined by next-generation sequencing harbored no CIC missense mutations. Using DNA methylation profiling, one CD31+ CIC-rearranged sarcoma was clustered with CD31− CIC-rearranged sarcomas, but distant from angiosarcomas. When compared with epithelioid angiosarcomas lacking CIC rearrangements, ERG+/CD31+ CIC-rearranged sarcomas were distinguished by focal myxoid change and the entire lack of vasoformative architecture. The angiosarcomas were characterized by uniform strong expression of ERG and CD31, but none of them were found positive for ETV4 or nuclear WT1. Heterogeneous ERG/CD31 co-expression in a subset of CIC-rearranged sarcoma is a clinically relevant pitfall for angiosarcoma, as these two diseases are treated differently.
Similar content being viewed by others
Introduction
CIC-rearranged sarcoma is a rare high-grade sarcoma that is defined by the rearrangement of the CIC gene (19q13) to various partners, with >90% of cases harboring CIC-DUX4 via t(4;19)(q35;q13) or t(10;19)(q26;q13).1,2 CIC-rearranged sarcomas occur in a wide age range but display peak incidence in young adults. Most examples arise from soft tissues, but visceral involvement (e.g., the brain or kidneys) has occasionally been reported. These tumors are histologically characterized by lobulated or diffuse growth of minimally pleomorphic, mitotically active, round to epithelioid cells with vesicular chromatin and prominent nucleoli.3 Diagnostically helpful immunophenotypes include positive expression of ETV4 and nuclear WT1 as well as negative expression of NKX2.2.3,4,5 CIC-rearranged sarcoma is resistant to chemotherapy and associated with significantly worse survival than Ewing sarcomas.3,6
Overall, CIC-rearranged sarcomas are undifferentiated. Recently, however, we encountered a CIC-DUX4 sarcoma that was initially diagnosed as angiosarcoma based on the heterogeneous co-expression of transcription factor ERG and surface adhesion molecule CD31, a widely accepted combination of markers for vascular endothelial differentiation.7,8 This case reminded us of a recent discovery by Huang et al.,9 who found CIC rearrangements in three tumors that were diagnosed as epithelioid angiosarcomas. The tumors occurred in adult females and involved soft tissues and the kidney. They consisted of diffuse proliferation of round or epithelioid cells with a lack of vasoformation, and demonstrated diffuse strong expression of ERG and CD31. CD34 expression was observed in one case, CIC-LEUTX fusion in one case in which the fusion partner was available, and concomitant CIC missense mutation was found in two of the three cases.9
CIC-rearranged sarcomas are known to frequently express ERG;10,11 however, the incidence of combined CD31 expression has not been studied well. Therefore, we conducted a systematic investigation in a relatively large cohort of well-characterized CIC-rearranged sarcomas and correlated their immunoprofiles with clinicopathological characteristics. We also studied epithelioid angiosarcomas that lacked CIC rearrangements for comparison.
Materials and methods
Cases
Based on availability of tissue for further immunostaining, we retrieved 30 samples of nearly-consecutively accessioned CIC-rearranged sarcomas, including the index case, originating from 30 different patients, from the pathology archive (1996–2020) of the National Cancer Center Hospital. Personal consultation cases were excluded. Clinical, immunohistochemical, and molecular data were obtained from medical records and pathology reports. These cases were identified during our previous research3,12 or diagnostic practice, when the presence of CIC rearrangement was confirmed in all cases, using various methods including CIC break-apart fluorescence in situ hybridization (FISH), reverse transcription polymerase chain reaction, Sanger sequencing, and/or next-generation sequencing (NGS). Nineteen cases harbored CIC-DUX4 fusion, while the remaining 11 cases showed CIC-rearrangements as per FISH, with no further work-ups performed to identify fusion partners. Nineteen cases were previously published by our group.3,12 As a comparison cohort, we retrieved ten archival samples of epithelioid angiosarcoma with epithelioid components comprising >90% of the tumor volume. Nine of these samples were tested negative for CIC rearrangement by FISH, while hybridization failed in the remaining case.
Immunohistochemistry
Immunohistochemistry was performed on 4-μm-thick formalin-fixed, paraffin-embedded tumor sections. All CIC-rearranged sarcomas were stained with ERG and CD31 antibodies if not already conducted at original diagnosis. The primary antibodies were ERG (EPR3864, dilution 1:2000; Abcam, Cambridge, UK) and CD31 (JC/70A, dilution 1:50; Dako, Glostrup, Denmark). CIC-rearranged sarcomas were previously tested for ETV4 and WT1 using ETV4 (PEA3; 16, dilution 1:50; Santa Cruz Biotechnology, Dallas, TX, USA) and WT1 (6F-H2, dilution 1:50; Dako) antibodies. Epithelioid angiosarcoma cases in the comparison cohort were also tested for ERG, CD31, ETV4, and WT1 if not already conducted at original diagnosis. Heat-induced epitope retrieval was performed, and the reactions were detected with the EnVision system (Dako). Diaminobenzidine was used as the chromogen, and hematoxylin was used as the counterstain.
For ERG and CD31, the extent of staining was recorded as diffuse (3+; >50%), focal (2+; 50% to >10%), rare (1+; 10% to >0%), or negative (0; 0%); the intensity of staining was recorded as strong, moderate, or weak: strong expression (3+) was as intense as the reactivity to the non-neoplastic vascular endothelium included in the slides, moderate expression (2+) was slightly weaker than endothelial staining but easy to recognize at low-power magnification, and weak expression (1+) was weaker than endothelial staining and clearly visible only at high-power magnification. For ERG, only moderate or strong (2+ or 3+) nuclear expression was considered positive. For CD31, any degree of definitive reactivity in the tumor cell membrane was considered positive, after carefully excluding reactivity in intra-tumoral histiocytes or other immune cells, which is a known pitfall.13 These criteria of ERG/CD31 interpretation were adopted in light of the substantive risk of confusion with endothelial tumors. Based on our experience, weak ERG expression is relatively common in non-endothelial tumors; therefore, it was excluded. In contrast, given the high specificity of CD31, any definitive membranous positivity of this marker in tumor cells could prompt consideration of endothelial tumors. For ETV4, moderate or strong nuclear staining in ≥30% of tumor cells was considered positive, as previously published.12 For WT1, nuclear reactivity of any degree was considered positive.
CIC mutation assays
One case (case 1) was clinically tested using FoundationOne® CDx, which covers CIC. Adequate tissue was available for the use of the NCC Oncopanel Ped assay in three additional ERG+/CD31+ CIC-rearranged sarcomas (cases 3, 4, and 8). This assay is designed to identify mutations/amplifications in 211 genes, including all exons of CIC (Supplementary Table 1).
DNA methylation analysis
Additional material was available from one ERG+/CD31+ CIC-rearranged sarcoma (case 4) for DNA methylation analysis. Three CD31− CIC-rearranged sarcomas were also analyzed as controls. The analysis was performed as previously described,14 using Infinium MethylationEPIC BeadChips (EPIC, Illumina). The Deutsche Krebsforschungszentrum (DKFZ) methylation profiling classifier was used (https://www.molecularneuropathology.org/msp/) to assign subtype scores for each tumor.15 Unprocessed IDAT files for 1077 samples, which include 37 angiosarcoma and 11 CIC-rearranged sarcoma samples among other tumor types, were downloaded from the NCBI Gene Expression Omnibus under the accession number GSE140686 and used as a reference. To perform unsupervised non-linear dimension reduction, the 10,000 most variable probes (according to standard deviation) were selected among 1077 reference samples. The t-distributed stochastic neighbor embedding (t-SNE) plots for our four CIC-rearranged sarcomas and 1077 reference samples were made using the Rtsne package (version 0.15).
Statistical analysis
All statistical analyses were performed using EZR (version 1.37; Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (version 3.4.1; The R Foundation for Statistical Computing, Vienna, Austria).16 The Fisher exact test was used to analyze the categorical data, and the Mann–Whitney U test was used to analyze the continuous data. Overall survival, which was measured from the date of the initial biopsy or resection, was determined using the Kaplan–Meier method, and the difference in survival was compared using the log-rank test. p < 0.05 was considered statistically significant.
Results
Recurrent co-expression of ERG and CD31 in CIC-rearranged sarcomas
Among the 30 CIC-rearranged sarcomas tested, 15 (50%) showed moderate or strong ERG expression in <5–100% of tumor cells. The remaining cases displayed either weak (5 cases, 17%) or negative (10 cases, 33%) ERG expression. Among the 15 ERG-positive cases, nine were variably positive for CD31, with four showing diffuse (>50%) CD31 expression. None of the cases showed a CD31+ERG− profile.
Characterization of ERG+/CD31+ CIC-rearranged sarcomas
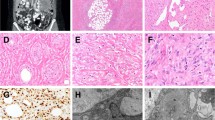
The findings of the nine ERG+/CD31+ CIC-rearranged sarcomas are summarized in Table 1. The patients included five men and four women with a median age of 29 years. The tumors occurred in superficial soft tissue in five cases, deep soft tissue in three cases, and the heart in one case. All nine tumors displayed classic histology of CIC-rearranged sarcoma and were characterized by lobulated or diffuse proliferation of minimally pleomorphic round cells with nucleoli of variable size and brisk mitoses (Fig. 1A, B). Epithelioid cells and spindle cells were focally observed in a subset of cases. Focal myxoid changes were present in six cases (Fig. 1C). The tumor cytoplasm was clear to eosinophilic, and clearing occasionally assumed vacuolated appearance in seven cases, mimicking intracytoplasmic lumina, especially when associated with hemorrhage (Fig. 1D). None of the cases exhibited true vasoformative architecture; however, five cases showed focal areas of clefts or small cysts associated with hemorrhage, which mimicked vascular spaces (Fig. 1E, F).
The tumors showed lobulated growth in the background of sclerotic stroma (A), comprising minimally pleomorphic round to epithelioid cells with visible nucleoli and clear to eosinophilic cytoplasm (B). Myxoid changes in association with reticular tumor growth, a specific finding not observed in epithelioid angiosarcoma, was present in many cases (C). Focal cytoplasmic vacuoles were observed in most cases (D) and thus were not helpful for the distinction from angiosarcoma. In some cases, the focal presence of hemorrhagic cysts or clefts mimicked vessel formation (E, F).
ERG and CD31 were expressed in a variable intensity and extent, with strong expression observed in seven and four cases, respectively, and diffuse expression in four cases each (Fig. 2A–G). The expression was heterogeneous rather than uniformly diffuse and strong. The areas expressing ERG and CD31 overlapped significantly, but the areas with strong expression for each marker did not always match. Seven cases (cases 1–7) were tested for CD34 and all were found negative. One case (case 5) was tested for FLI1 and was found positive. All cases expressed ETV4 and/or nuclear WT1 (Fig. 2H, I), with seven cases co-expressing both markers. CIC-DUX4 fusion transcript was confirmed in seven cases, including three that showed negative CIC FISH results.12 CIC rearrangement was confirmed by FISH in the remaining two cases.
The tumors expressed CD31 in a heterogeneous manner (A strongly and moderately stained cells are admixed, B strongly, weakly, and negatively stained cells are admixed). Higher magnification view of a spectrum of CD31 expression in CIC-rearranged sarcomas (C, D marked staining; E, F more limited expression). Similarly, the tumors expressed ERG in a heterogeneous manner (G strongly and moderately stained cells are admixed). All cases were positive for ETV4 (H) and/or nuclear WT1 (I).
Reflective of the retrospective nature of the study, these nine patients originally received variable diagnoses and were treated accordingly. Notably, three patients (cases 1, 2, and 5) were diagnosed with epithelioid angiosarcoma at least once during their disease courses, a diagnosis that was ultimately revised. Case 1 (14-year-old male) presented with a mass in the scalp skin, showing lobulated round to epithelioid cells with widespread, mainly strong co-expression of ERG and CD31. Case 2 (29-year-old female) presented with cardiac tamponade. The mass resected from the right ventricle showed prominent epithelioid morphology, widespread cytokeratin AE1/AE3 expression, and diffuse strong ERG expression along with rare weak CD31 expression. In case 5 (21-year-old male), the primary tumor in the diaphragm was diagnosed as high-grade small round cell sarcoma; however, the patient subsequently experienced two episodes of highly hemorrhagic brain metastases, whose biopsies displayed epithelioid cells with ERG and CD31 co-expression. These three patients accordingly received paclitaxel, which is a preferred regimen for angiosarcomas, but none displayed significant treatment effects. Four patients succumbed to the disease within 6–11 months.
CIC mutation assays
None of the four ERG+/CD31+ CIC-rearranged sarcomas tested by NGS harbored CIC missense mutations.
DNA methylation analysis
One ERG+/CD31+ CIC-rearranged sarcoma was predicted as “methylation class small blue round cell tumor with CIC alteration” by the DKFZ classifier, with a moderate calibrated score of 0.85. All three CD31− CIC-rearranged sarcomas were also predicted as “methylation class small blue round cell tumor with CIC alteration” with low to high scores (scores 0.35–0.98). The t-SNE analysis showed that all four cases clustered together with CIC-rearranged sarcoma reference samples, while being distant from angiosarcomas (Fig. 3).
The reference cohort (n = 1077, including 37 angiosarcoma and 11 CIC-rearranged sarcoma samples among other tumor types) and our CIC-rearranged sarcoma samples (n = 4; one CD31+ and three CD31− cases) were plotted using t-distributed stochastic neighbor embedding (t-SNE) dimensionality reduction. Our tumors are indicated as red squares. The color code of the DNA methylation classes was the same as in Koelsche et al.15 All of our four tumors were plotted together with the CIC-rearranged sarcoma reference samples, while being distant from angiosarcomas. Brown dots in a circle represent 37 reference angiosarcomas and blue dots in a square represent 11 reference CIC-rearranged sarcomas whose ERG/CD31 status is unknown.
Comparison with CIC-rearranged sarcomas lacking ERG/CD31 co-expression
When the nine ERG+/CD31+ CIC-rearranged sarcomas were compared with the remaining 21 CIC-rearranged sarcomas lacking co-expression, no significant differences were identified regarding age, sex, tumor site, and histology. Hemorrhagic cysts and cytoplasmic vacuoles were focally identified in many of these 21 cases. However, ERG+/CD31+ CIC-rearranged sarcomas were significantly smaller in size (p = 0.0332; Mann–Whitney U test), and superficial tumors were overrepresented (p = 0.0318; Fisher exact test). The outcomes of these two cohorts were not significantly different (p = 0.918; Kaplan–Meier method and log-rank test).
Comparison with the epithelioid angiosarcoma cohort
The cohort of ten epithelioid angiosarcomas was compared with the nine cases of ERG+/CD31+ CIC-rearranged sarcomas. A clinicopathological summary of the epithelioid angiosarcomas is provided in Table 2. Angiosarcoma occurred in eight men and two women with a median age of 42.5 years (range: 16–87 years). Most epithelioid angiosarcomas in this series showed solid sheets of epithelioid cells (Fig. 4A). Overall, the tumor cells tended to show a larger size, more prominent nucleoli, and more amphophilic cytoplasm compared to those in CIC-rearranged sarcomas, although the cytological differences were sometimes subtle. Eight epithelioid angiosarcomas focally harbored intracytoplasmic vacuoles, some of which contained erythrocytes; however, similar findings were also noted in most CIC-rearranged sarcomas. Unlike CIC-rearranged sarcomas, eight angiosarcomas demonstrated focal evidence of vasoformative architecture in the form of irregular, sometimes anastomosing vessels, particularly at the tumor periphery (Fig. 4B). Conversely, none of the angiosarcomas exhibited the focal myxoid change and reticular growth that was frequently observed in CIC-rearranged sarcomas. All 10 epithelioid angiosarcomas immunohistochemically showed uniformly diffuse and strong expression of ERG and CD31 (Fig. 4C, D), which was different from the heterogeneous expression in CIC-rearranged sarcomas. In contrast, none of the angiosarcomas were positive for ETV4 or nuclear WT1 (Fig. 4E, F), although cytoplasmic WT1 expression was common in angiosarcomas (eight cases, 80%). Paclitaxel was administered in six cases, none of which exhibited a significant response. Nine patients succumbed to the diseases within 2–38 months. The overall survival of epithelioid angiosarcomas was not significantly different from that of the ERG+/CD31+ CIC-rearranged sarcomas (p = 0.279; Kaplan–Meier method and log-rank test). Table 3 compares the histological findings between nine cases of ERG+/CD31+ CIC-rearranged sarcomas and ten cases of epithelioid angiosarcomas.
The tumors predominantly consisted of sheets of large epithelioid cells with amphophilic cytoplasms and vesicular nuclei with large nucleoli (A). A minor component of irregular vascular formation lined by malignant cells was often observed at the periphery of the tumor (B). All tumors expressed ERG (C) and CD31 (D) in a homogeneous diffuse strong manner. Epithelioid angiosarcomas were uniformly negative for ETV4 (E) and nuclear WT1 (F notice cytoplasmic WT1 expression, which should not be confused with diagnostically relevant nuclear labeling).
Discussion
The present study demonstrated that a subset of CIC-rearranged sarcomas at least focally co-expressed ERG and CD31. This is a widely accepted combination of vascular endothelial markers, although neither is entirely specific, as CD31 is expressed in immune cells including histiocytes/macrophages13 and ERG is expressed in myeloid cells, chondrogenic tumors, and a subset of other non-vascular tumors with various lineages.17,18,19 The ERG/CD31 co-expression forms a spectrum in CIC-rearranged sarcomas, spanning from weak or focal to strong or diffuse rather than making two discrete groups. These tumors were otherwise similar to the CIC-rearranged sarcomas that lacked co-expression, except that smaller superficial tumors were overrepresented. Seven of the co-expressing tumors harbored canonical CIC-DUX4 fusions, and one case undergoing DNA methylation profiling clustered with CD31– CIC-rearranged sarcomas, being distant from angiosarcoma. Altogether, our data suggest that ERG and CD31 expression is an inherent phenotypic variation observed in a subset of CIC-rearranged (otherwise undifferentiated) sarcomas, rather than defining a separate disease.
This is a realistic pitfall for the diagnosis of angiosarcoma, because in a clinical context of high-grade sarcoma, which is often evaluated upon small biopsy, even the slightest hint of differentiation tends to be sought in an attempt to classify tumors. Indeed, three of our cases were originally interpreted as angiosarcoma, even though none of them showed uniformly diffuse and strong ERG/CD31 co-expression. In addition, their clinical pictures were confusing and overlapping with angiosarcoma, including the involvement of scalp skin (case 1), right ventricular mass with cardiac tamponade (case 2), and massively hemorrhagic brain metastases (case 5). Comparison with epithelioid angiosarcomas lacking CIC rearrangements allowed identification of several helpful findings for differential diagnosis. Most angiosarcomas at least focally exhibited compelling vascular channel formation, which is in agreement with a previous study.20 Although hemorrhagic cysts or clefts were focally observed in many CIC-rearranged sarcomas, they had a jagged contour rather than rigid vascular spaces. In contrast, focal myxoid change with reticular growth was specific to CIC-rearranged sarcomas. Immunohistochemically, ERG and CD31 were co-expressed in all epithelioid angiosarcomas in a diffuse, strong, and homogeneous manner, unlike the heterogeneous and usually multifocal expression in CIC-rearranged sarcomas. In addition, none of the epithelioid angiosarcomas were positive for ETV4 or nuclear WT1, although cytoplasmic WT1 expression was common in angiosarcomas. We therefore believe that the histology and immunoprofile should distinguish two diseases in most instances, while molecular genetic analysis of CIC fusion may be necessary only in challenging cases, with the caveat that FISH analysis may miss a minority of cases with rearrangements.12,21
CIC-rearranged sarcomas and angiosarcomas are treated differently, particularly at an advanced stage. Although the standard therapy for CIC-rearranged sarcomas has not been established, patients typically receive similar regimens to those of Ewing sarcomas.22 In contrast, patients with angiosarcoma often receive taxane-based treatment (e.g., weekly paclitaxel).23 In our study, three CIC-rearranged sarcoma patients, who originally received the diagnosis of angiosarcoma, were treated with paclitaxel, but none responded. However, six patients with a final diagnosis of epithelioid angiosarcoma who were treated with paclitaxel did not respond well either, and both cohorts in this study had comparably poor survival, making it impossible to determine whether the classification had a significant impact on patient prognosis.
Given recurrent ERG/CD31 co-expression in CIC-rearranged sarcoma, it is possible that the three tumors reported by Huang et al.9 might also belong to CIC-rearranged sarcoma. In fact, the RNA expression profiles of two of them clustered with those of CIC-DUX4 undifferentiated sarcomas, but were distant from those of conventional angiosarcomas.9 However, the interpretation remains indeterminate, because the findings did not overlap completely between the two studies. First, unlike the sarcomas reported by Huang et al., the tumors studied in this paper never showed diffuse and strong ERG/CD31 co-expression. Second, none of the tumors with ERG/CD31 co-expression, as studied in this paper, had CIC missense mutation. Third, none of the presently reported tumors harbored CIC-LEUTX fusion. Lastly, none of our tumors were tested positive for CD34. Because CIC missense mutations are recurrent even in a subset of angiosarcomas lacking CIC rearrangement,9 it is possible that CIC missense mutation and fusion might have a synergistic effect to allow enhanced expression of ERG and CD31. The rare CIC fusion partnership with LEUTX might also drive somewhat different phenotypes from those associated with CIC-DUX4. In this regard, it is interesting that none of the five CIC-LEUTX-positive tumors reported to date received the CIC-rearranged sarcoma diagnosis. Similar to the case reported by Huang et al.,9 one tumor with CIC-LEUTX was reported as primary angiosarcoma of the central nervous system (CNS).24 The remaining three tumors with CIC-LEUTX, also in the CNS, showed neuroepithelial phenotypes with variable expression of glial fibrillary acidic protein and synaptophysin and were interpreted as anaplastic ganglioglioma, anaplastic pleomorphic xanthoastrocytoma, and CNS embryonal tumor.25,26 Our cohort included two ERG+/CD31+ CIC-rearranged sarcomas with unknown fusion partners; however, neither tumor phenotypically matched angiosarcoma.
Membranous CD31 expression in tumors of non-endothelial/non-immune lineages is extremely limited, and previous studies have documented positive expression in a small subset of carcinomas27,28,29 and round cell sarcomas.30 In the latter study, Nicholson et al.30 identified focal membranous CD31 expression in four out of 85 Ewing sarcoma/primitive neuroectodermal tumors (4.7%), three in the soft tissues and one in the brain. In one of them, artifactual dyscohesion and clefting originally suggested a diagnosis of angiosarcoma. Interestingly, the published illustrations of these cases showed less uniform nuclear size as well as more ample cytoplasm than classic Ewing sarcomas. These histological features suggest the possibility that they may represent CIC-rearranged sarcomas,3 although none of these cases were molecularly characterized at the time of their publication in 2000.
In conclusion, a subset of CIC-rearranged sarcomas co-expressed ERG and CD31 in a heterogeneous pattern with a spectrum of reactivity, representing a diagnostic pitfall. These tumors showed the otherwise classic histomorphology and immunoprofile of this sarcoma type, with some displaying the typical CIC-DUX4 fusion and the overlapping DNA methylation profile with the CD31− subset. Histological examination should suffice to distinguish epithelioid angiosarcoma from CIC-rearranged sarcoma in most instances. The distinction from angiosarcoma would be clinically important because the treatment regimens are often different, although the prognosis is similarly poor for both diseases.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request and IRB approval.
References
Italiano, A., Sung, Y. S., Zhang, L., Singer, S., Maki, R. G., Coindre, J. M. et al. High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosomes Cancer 51, 207–218 (2012).
Kawamura-Saito, M., Yamazaki, Y., Kaneko, K., Kawaguchi, N., Kanda, H., Mukai, H. et al. Fusion between CIC and DUX4 up-regulates PEA3 family genes in Ewing-like sarcomas with t(4;19)(q35;q13) translocation. Hum Mol Genet 15, 2125–2137 (2006).
Yoshida, A., Goto, K., Kodaira, M., Kobayashi, E., Kawamoto, H., Mori, T. et al. CIC-rearranged Sarcomas: A Study of 20 Cases and Comparisons With Ewing Sarcomas. Am J Surg Pathol 40, 313–323 (2016).
Hung, Y. P., Fletcher, C. D. & Hornick, J. L. Evaluation of ETV4 and WT1 expression in CIC-rearranged sarcomas and histologic mimics. Mod Pathol 29, 1324–1334 (2016).
Le Guellec, S., Velasco, V., Perot, G., Watson, S., Tirode, F. & Coindre, J. M. ETV4 is a useful marker for the diagnosis of CIC-rearranged undifferentiated round-cell sarcomas: a study of 127 cases including mimicking lesions. Mod Pathol 29, 1523–1531 (2016).
Antonescu, C. R., Owosho, A. A., Zhang, L., Chen, S., Deniz, K., Huryn, J. M. et al. Sarcomas With CIC-rearrangements Are a Distinct Pathologic Entity With Aggressive Outcome: A Clinicopathologic and Molecular Study of 115 Cases. Am J Surg Pathol 41, 941–949 (2017).
DeYoung, B. R., Swanson, P. E., Argenyi, Z. B., Ritter, J. H., Fitzgibbon, J. F., Stahl, D. J. et al. CD31 immunoreactivity in mesenchymal neoplasms of the skin and subcutis: report of 145 cases and review of putative immunohistologic markers of endothelial differentiation. J Cutan Pathol 22, 215–222 (1995).
Miettinen, M., Lindenmayer, A. E. & Chaubal, A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens--evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol 7, 82–90 (1994).
Huang, S. C., Zhang, L., Sung, Y. S., Chen, C. L., Kao, Y. C., Agaram, N. P. et al. Recurrent CIC Gene Abnormalities in Angiosarcomas: A Molecular Study of 120 Cases With Concurrent Investigation of PLCG1, KDR, MYC, and FLT4 Gene Alterations. Am J Surg Pathol 40, 645–655 (2016).
Smith, S. C., Buehler, D., Choi, E. Y., McHugh, J. B., Rubin, B. P., Billings, S. D. et al. CIC-DUX sarcomas demonstrate frequent MYC amplification and ETS-family transcription factor expression. Mod Pathol 28, 57–68 (2015).
Specht, K., Sung, Y. S., Zhang, L., Richter, G. H., Fletcher, C. D. & Antonescu, C. R. Distinct transcriptional signature and immunoprofile of CIC-DUX4 fusion-positive round cell tumors compared to EWSR1-rearranged ewing sarcomas: further evidence toward distinct pathologic entities. Genes Chromosomes Cancer 53, 622–633 (2014).
Yoshida, A., Arai, Y., Kobayashi, E., Yonemori, K., Ogura, K., Hama, N. et al. CIC break-apart fluorescence in-situ hybridization misses a subset of CIC-DUX4 sarcomas: a clinicopathological and molecular study. Histopathology 71, 461–469 (2017).
McKenney, J. K., Weiss, S. W. & Folpe, A. L. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol 25, 1167–1173 (2001).
Yoshida, A., Arai, Y., Satomi, K., Kubo, T., Ryo, E., Matsushita, Y. et al. Identification of novel SSX1 fusions in synovial sarcoma. Mod Pathol 35, 228–239 (2022).
Koelsche, C., Schrimpf, D., Stichel, D., Sill, M., Sahm, F., Reuss, D. E. et al. Sarcoma classification by DNA methylation profiling. Nat Commun 12, 498 (2021).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48, 452–458 (2013).
Miettinen, M., Wang, Z. F., Paetau, A., Tan, S. H., Dobi, A., Srivastava, S. et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol 35, 432–441 (2011).
Shon, W., Folpe, A. L. & Fritchie, K. J. ERG expression in chondrogenic bone and soft tissue tumours. J Clin Pathol 68, 125–129 (2015).
Wang, W. L., Patel, N. R., Caragea, M., Hogendoorn, P. C., Lopez-Terrada, D., Hornick, J. L. et al. Expression of ERG, an Ets family transcription factor, identifies ERG-rearranged Ewing sarcoma. Mod Pathol 25, 1378–1383 (2012).
Fletcher, C. D., Beham, A., Bekir, S., Clarke, A. M. & Marley, N. J. Epithelioid angiosarcoma of deep soft tissue: a distinctive tumor readily mistaken for an epithelial neoplasm. Am J Surg Pathol 15, 915–924 (1991).
Kao, Y. C., Sung, Y. S., Chen, C. L., Zhang, L., Dickson, B. C., Swanson, D. et al. ETV transcriptional upregulation is more reliable than RNA sequencing algorithms and FISH in diagnosing round cell sarcomas with CIC gene rearrangements. Genes Chromosomes Cancer 56, 501–510 (2017).
Palmerini, E., Gambarotti, M., Ratan, R., DuBois, S., Nathenson, M. J., Italiano, A. et al. Graceful project: a global collaboration on CIC-DUX4, BCOR-CCNB3, high grade undiffifferentiated round cell sarcoma (URCS) Proceedings of the CTOS Annual Meeting 2019; Tokyo, Japan. 13–16 November 2019. (2019).
Penel, N., Bui, B. N., Bay, J. O., Cupissol, D., Ray-Coquard, I., Piperno-Neumann, S. et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol 26, 5269–5274 (2008).
Noch, E., Nacev, B., Chan, J., Wolden, S., Tap, W., Antonescu, C. et al. A 43 year-old woman with primary central nervous system angiosarcoma with CIC-LEUTX gene rearrangement (P3.6-017). Neurology 92 (2019).
Hu, W., Wang, J., Yuan, L., Zhang, X., Ji, Y., Song, C. et al. Case Report: A Unique Case of Pediatric Central Nervous System Embryonal Tumor Harboring the CIC-LEUTX Fusion, Germline NBN Variant and Somatic TSC2 Mutation: Expanding the Spectrum of CIC-Rearranged Neoplasia. Front Oncol 10, 598970 (2020).
Lake, J. A., Donson, A. M., Prince, E., Davies, K. D., Nellan, A., Green, A. L. et al. Targeted fusion analysis can aid in the classification and treatment of pediatric glioma, ependymoma, and glioneuronal tumors. Pediatr Blood Cancer 67, e28028 (2020).
Ortiz-Hidalgo, C., Torres, J. E., Cuesta-Mejias, T. & Mendoza-Ramon, H. CD31 with strong membrane-based immunoreactivity in ductal carcinoma of the breast. Appl Immunohistochem Mol Morphol 8, 334–335 (2000).
Sapino, A., Bongiovanni, M., Cassoni, P., Righi, L., Arisio, R., Deaglio, S. et al. Expression of CD31 by cells of extensive ductal in situ and invasive carcinomas of the breast. J Pathol 194, 254–261 (2001).
De Young, B. R., Frierson, H. F., Jr., Ly, M. N., Smith, D. & Swanson, P. E. CD31 immunoreactivity in carcinomas and mesotheliomas. Am J Clin Pathol 110, 374–377 (1998).
Nicholson, S. A., McDermott, M. B., DeYoung, B. R. & Swanson, P. E. CD31 immunoreactivity in small round cell tumors. Appl Immunohistochem Mol Morphol 8, 19–24 (2000).
Acknowledgements
The authors thank Sachiko Miura, Toshiko Sakaguchi, Chizu Kina, Hiroki Kakishima, and Hiroshi Chigira for their superb technical assistance.
Funding
This work was supported in part by JSPS KAKENHI (Grant Number JP21K06919, A.Y.) and the National Cancer Center Rare Cancer Grant (Award Number G007, A.Y.).
Author information
Authors and Affiliations
Contributions
A.Y. designed the study. N.K. and A.Y. conducted clinicopathological and immunohistochemical analyses. N.K., A.Y., Y.A., K.S., T.K., Y.M., H.M., T.U., T.S., K.I., and H.I. generated, analyzed, and interpreted molecular data. T.M., Y.Y., K.Y., and A.K. provided samples and data. N.K. and A.Y. wrote the manuscript with contributions from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board of the National Cancer Center Hospital, Tokyo, Japan (No.2014-089).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kojima, N., Arai, Y., Satomi, K. et al. Co-expression of ERG and CD31 in a subset of CIC-rearranged sarcoma: a potential diagnostic pitfall. Mod Pathol 35, 1439–1448 (2022). https://doi.org/10.1038/s41379-022-01078-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01078-8
This article is cited by
-
Myxoid liposarcoma with nuclear pleomorphism: a clinicopathological and molecular study
Virchows Archiv (2024)