Abstract
Risk stratification of gastrointestinal stromal tumors (GISTs) is based on experience with tumors of the stomach, small bowel, and rectum, which are far more common than GISTs of other sites. In this study from 47 institutions, we analyzed GISTs of the esophagus (n = 102), colon (n = 136), and appendix (n = 27) for their size, mitotic rate, morphology, and outcome to determine which criteria predict their behavior. Esophageal GISTs were small (median: 2.5 cm) with spindle cell morphology and a low mitotic rate (mean: 3.6/5 mm2). Twelve (12%) tumors progressed, including 11 with a mitotic rate >5/5 mm2 and one large (6.8 cm) GIST with a mitotic rate of 2/5 mm2. Colonic GISTs were smaller (median: 1.4 cm) and presented with abdominal pain or bleeding in 29% of cases. Most (92%) were composed of spindle cells with a mean mitotic rate of 4.6/5 mm2. Sixteen (12%) tumors progressed: 14 had mitotic rates >5/5 mm2, and two were >5.0 cm with a mitotic rate <5/5 mm2. All but one appendiceal GIST measured <2.0 cm. These tumors were composed of spindle cells with low mitotic rates (<5/5 mm2), and none progressed. Our results suggest that progression risk among esophageal and colonic GISTs is associated with increased mitotic activity (>5/5 mm2) and size >5.0 cm. These findings support the use of size and mitotic rate for prognostication of GISTs in these locations, similar to tumors of the stomach, small bowel, and rectum.
Similar content being viewed by others
Introduction
Gastrointestinal stromal tumor (GIST), which possesses phenotypic features of interstitial cells of Cajal, is the most common malignant mesenchymal tumor of the gastrointestinal tract1. The annual incidence of GIST was estimated to be 0.7 per 100,000 persons in the United States during the time period 2001–2005, inclusively2. GISTs most frequently occur in the stomach (60.3%), followed by the small bowel (33.2%), rectum (3.1%), and colon (2.9%)3. They are rare in the esophagus (0.7%) and even rarer in the appendix3.
GISTs comprise tumors with variable malignant potential. Incidentally discovered microscopic GISTs of the stomach almost never progress, whereas large and/or mitotically active GISTs are at high risk of disease progression, especially when they occur in the small bowel. Risk assessment is important in identifying high-risk patients who may benefit from adjuvant treatment. Studies have demonstrated that tumor site, size, and mitotic rate can be used to stratify risk of progression in GISTs4,5,6,7,8. Several nomograms have been proposed for purposes of prognostication9, but the table published by the College of American Pathologists (CAP) in their Protocol for the Examination of Resection Specimens From Patients With Gastrointestinal Stromal Tumors is likely the most widely used10. This table provides site-specific risk assessment for GISTs arising in the stomach, small bowel, and rectum and was adapted from previously published recommendations6.
Although similar criteria have been applied to GISTs of the esophagus, colon, and appendix, these tumors have been incompletely studied because they are quite uncommon. In this multi-institutional study, we analyzed a large cohort of GISTs arising from the esophagus, colon, and appendix to evaluate their clinicopathologic features and determine whether size and mitotic rate predict risk of progression, similar to GISTs of other sites.
Materials and methods
Primary GISTs of the esophagus, colon, and appendix were identified from the pathology archives of 47 institutions in the United States and Canada. The components of the study conducted at each institution were approved by their respective institutional review boards. Existing glass slides were retrieved for each case and reviewed by surgical pathologists with expertise in gastrointestinal mesenchymal tumors at each institution. Confirmation of the diagnosis via histologic appearance combined with immunohistochemistry for KIT or DOG1, or mutation of KIT or PDGFRA, was required for inclusion. Cases consisting of biopsy and/or polypectomy samples were included when patients did not undergo surgical resection. Neoadjuvantly or adjuvantly treated cases were also included. Tumors arising in the gastroesophageal junction, rectosigmoid colon, or rectum were excluded. Cases were also excluded when patients had prior or concomitant GISTs of the stomach, small bowel, or rectum, or when any criteria relating to risk of progression (tumor size, mitotic rate, patient follow-up) were lacking. A minimum follow-up of one month was required unless evidence was available of distant metastatic disease at the time of surgery or of progression within one month.
Clinical, laboratory, and demographic data were retrieved from electronic medical records at each institution. These included patient age, sex, presenting symptoms, imaging findings, treatment history, and outcome. Tumors were assessed for size, mitotic rate per 5 mm2, morphology (spindle cell, epithelioid, or mixed), necrosis, adequacy of resection, immunohistochemical staining results, and pathologic stage. Tumor size was generally measured grossly; it was determined based on imaging studies when cases consisted of biopsy material alone or direct measurement of material on glass slides when endoscopically resected polyps were completely excised. Disease progression was defined as metastatic disease (including disease at initial presentation) and/or GIST-related death. Immunohistochemical stains were performed using each institution’s local protocols; no additional immunohistochemical stains were performed as part of this study.
Associations between clinicopathologic features and disease progression were examined using landmark analysis at 1-year progression-free survival (PFS), stratified by specimen type (biopsy vs resection) and treatment (neoadjuvant and adjuvant therapy). Tests were two-sided, and a P value < 0.05 was considered statistically significant. Not all noncrucial clinicopathologic data were available for every patient, so analysis of each parameter was conducted on the cases with available information. Kaplan–Meier curves were generated for esophageal and colonic GISTs. Cox proportional hazards were performed on the combined esophageal, colonic, and appendiceal GISTs stratified by treatment regimen (biopsy only vs. resection and administration of neoadjuvant or adjuvant therapy). Necrosis was not included in the model because of the confounding effect of neoadjuvant therapy in some cases. Tumor morphology was dichotomized as purely spindled vs. either epithelioid or mixed, since very few cases were purely epithelioid. Otherwise, all variables (including anatomic site) were represented in the initial multivariable model and removed in stepwise fashion in order of |z-score| until all remaining variables were statistically significant at α = 0.05.
Results
We initially identified 337 cases for the study. Of these, 72 were excluded based on the failure to meet aforementioned criteria. The remaining 265 cases were deemed valid for inclusion.
Esophageal GISTs
There were 102 esophageal GISTs, including 13 biopsy cases (Fig. 1). Their clinicopathologic features are summarized in Table 1. All patients were adults, with a median age of 67 years (range: 27–83) and a male predominance (male:female ratio of 1.7:1). Half of the patients had esophageal symptoms, most commonly dysphagia and odynophagia (30%), followed by gastroesophageal reflux (8%) and epigastric pain (4%). Asymptomatic tumors were incidentally identified in esophagogastrectomy specimens or discovered by imaging or endoscopy. Most tumors (86%) were located in the distal esophagus, and they tended to be small (median: 2.5 cm, range: 0.1–12.0 cm). The mean mitotic rate was 3.6/5 mm2 (range: 0–32/5 mm2). A majority of the tumors (70, 70%) had a size of ≤5.0 cm and a mitotic rate of ≤5/5 mm2. Ninety-one (89%) tumors demonstrated purely spindle cell morphology, and 11 (11%) showed epithelioid or mixed morphology. Six (9%) treatment-naïve tumors showed necrosis. Molecular testing was performed on 25 tumors, including 16 with mutations or deletions of KIT exon 11, 4 with KIT exon 13 mutations, 1 with a KIT exon 9 mutation, and 4 without detectable mutations or deletions in KIT or PDGFRA. A germline NF1 mutation was identified in one patient who had neurofibromatosis type 1, but no molecular testing was performed on their esophageal GIST.
A Low-power view (4×) of a low-risk esophageal GIST, measuring <5.0 cm. B High-power view (40×) of the same GIST, showing spindled morphology and a low mitotic rate. C Low-power view (4×) of a high-risk esophageal GIST, measuring >5.0 cm. D High-power view (40×) of the same GIST, showing epithelioid morphology and a high mitotic rate.
The median follow-up was 33 months (range: 0–192). Twenty-nine patients received additional imatinib treatment (neoadjuvant in 10, adjuvant in 14, both in 5). Twelve (12%) patients demonstrated progression via tumor metastasis to the liver (n = 9) or peritoneal cavity (n = 3), including 9 patients who received imatinib. Two (2%) patients died of disease. The median interval to disease progression was 2 months (range: 0–117). Five patients had metastatic disease at the time of diagnosis. Landmark analysis at 1-year PFS was performed to evaluate the association of clinicopathologic features with disease progression. Of the 102 esophageal GIST cases, 76 (including 8 with progression) had follow-up data until the one-year landmark time, and were thus included in statistical analysis. Tumor size, mitotic rate, epithelioid morphology, and presence of tumor-related symptoms were significantly associated with disease progression on univariate analysis (Table 1), but margin status and tumor location in the esophagus (distal, mid, proximal) were not. Seven (88%) of the 8 patients with disease progression at 1-year landmark time had tumors that demonstrated a mitotic rate of >5/5 mm2, and the remaining patient (13%) had a tumor that showed 2 mitotic figures/5 mm2 but measured 6.8 cm. Overall, 39% of esophageal GISTs with mitotic rate >5/5 mm2 progressed at 1-year follow-up, as did 21% of tumors that were >5.0 cm in size. In contrast, only 2% of tumors with mitotic rate ≤5/5 mm2 and 6% of tumors ≤5.0 cm progressed at this time point. None of the 49 cases that measured ≤5.0 cm and had a mitotic rate ≤5/5 mm2 progressed at 1-year follow-up.
Colonic GISTs
There were 136 colonic GISTs (Fig. 2), including 24 sampled via biopsy and/or polypectomy alone. Their clinicopathologic features are summarized in Table 2. All patients were adults, with a median age of 66 years (range: 29–94). Men and women were similarly affected. A small proportion of patients presented with abdominal pain (12%), gastrointestinal bleeding (10%), or other symptoms potentially attributable to the tumor. For the remaining patients, GISTs were incidentally identified in colectomy specimens, during colonoscopy, or upon abdominal imaging. Most tumors were located in the left colon (52%), followed by the right (35%) and transverse (13%) colon. Colonic GISTs were relatively small (median size: 1.4 cm, range: 0.2–24.5 cm), with 46 cases smaller than 1.0 cm. They had a mean mitotic rate of 4.6/5 mm2 (range: 0–100/5 mm2), including 68 cases having no mitotic figures. A majority of the tumors (102, 75%) had a size of ≤5.0 cm and a mitotic rate of ≤5/5 mm2. The most common morphologic pattern was spindle cell type, which was seen in 124 (92%) of the cases. Twelve (12%) treatment-naïve tumors had necrosis. Molecular testing was performed in 17 tumors, including 10 with mutations or deletions involving KIT exon 11, 1 with a KIT exon 17 mutations, 2 with KIT exon 9 duplications, 1 with a PDGFRA exon 18 deletion, and 3 without detectable mutations or deletions in KIT or PDGFRA. One patient had a germline CHEK2 mutation, but no molecular testing was performed on their colonic GIST.
A Low-power view (4×) of a low-risk colonic GIST, measuring <5.0 cm. B High-power view (40×) of the same GIST, showing spindled morphology and a low mitotic rate. C Low-power view (4×) of a high-risk colonic GIST, measuring >5.0 cm. D High-power view (40×) of the same GIST, showing epithelioid morphology and a high mitotic rate.
The patients had a median follow-up of 36 months (range: 1–232). Twenty-four patients received imatinib (5 neoadjuvant, 16 adjuvant, 3 both), respectively. Sixteen (12%) patients developed metastases to the liver (n = 8), peritoneal cavity or pelvis (n = 8), or chest wall (n = 1), and a lymph node metastasis was identified in one patient who also had liver and peritoneal metastases. These 16 patients included 13 who received imatinib. Six (4%) patients died of their disease. The median interval to disease progression was 8.5 months (range: 0–132). Seven patients had metastasis at time of diagnosis. Landmark analysis at 1-year PFS was performed to evaluate the association of clinicopathologic features with disease progression. Of the 136 colonic GIST cases, 112 (including 11 with progression) had follow-up data until the 1-year landmark time, and were thus included in statistical analysis. Tumor size, mitotic rate, tumor necrosis, and presence of tumor-related symptoms were significantly associated with disease progression on univariate analysis (Table 2). Eight (22%) of the 36 patients with symptoms at presentation progressed at 1-year follow-up, while only 3 (4%) of the 76 patients without symptoms had disease progression. Nine (82%) of the 11 patients with disease progression at 1-year landmark time had tumors with a mitotic rate >5/5 mm2. The tumors in the remaining two patients (17%) had mitotic rates ≤5/5 mm2 but measured 9.5 cm and 14.0 cm, respectively. Overall, 47% of colonic GISTs with mitotic rate >5/5 mm2 and 39% with size >5.0 cm progressed at the 1-year follow-up, whereas none of the 80 tumors with mitotic rate ≤5/5 mm2 and size ≤5.0 cm showed progression at the same time point.
Appendiceal GISTs
There were 27 appendiceal GISTs (Fig. 3), and their clinicopathologic features are summarized in Table 3. The median patient age was 68 years (range: 34–83), and there was a female predominance (male:female ratio of 1:2). Four patients presented with acute appendicitis-like abdominal pain, two of whom had histologic acute appendicitis. In the remaining 23 patients, tumors were incidentally identified on imaging or colonoscopy, found during intraabdominal surgery, or encountered in right hemicolectomy specimens resected for other reasons. Appendiceal GISTs were quite small (median: 0.5 cm), with only four measuring larger than 1.0 cm (three tumors measured 1.3–1.5 cm and one tumor measured 4.5 cm). There was no association between symptoms and tumor size. Most tumors (22, 81%) showed no mitotic figures, three had 1/5 mm2, and two had 3/5 mm2. All appendiceal tumors showed spindle cell morphology, and none had tumor necrosis. Molecular testing was performed in two tumors; one showed in-frame deletion in KIT exon 11, and the other had no mutations in KIT, PDGFRA, or NF1. No germline mutations were identified in any of the patients.
Most patients underwent appendectomy, while six patients had right hemicolectomy or appendectomy along with partial cecectomy. None had additional surgery following the initial resection. Two tumors had a positive resection margin (one appendectomy and one appendectomy with partial cecectomy). The patients were followed up for a median length of 39 months (range: 1–156), and none demonstrated disease progression. Therefore, no statistical analysis was performed on this isolated cohort.
Overall risk assessment
As discussed above, the univariate landmark analyses showed that increased size and mitotic rate stratified by 5 per 5 mm2 were significantly associated with disease progression in esophageal GISTs (P = 0.004 and P < 0.0001, respectively) and in colonic GISTs (P < 0.0001 for both). Multivariate analysis was performed on the combined cohort of esophageal, colonic, and appendiceal GISTs since there were not enough cases with progression to run separate analyses by anatomic site. Tumor size >5 cm and mitotic rate >5/5 mm2 were identified as independent risk factors for progression (Table 4).
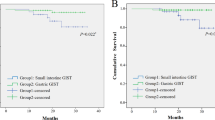
As size and mitotic rate are significantly associated with progression in GISTs at other locations, we created a risk-stratification table for esophageal, colonic, and appendiceal GISTs using all follow-up data (Table 5). A version of this table, structured in the same manner as the risk-stratification table for GISTs at other locations and additionally containing that known information6, is provided as Supplemental Table 1. We also created Kaplan–Meier curves to visualize the effect of size and mitotic rate on progression for esophageal GISTs (Fig. 4A, P < 0.0001) and colonic GISTs (Fig. 4B, P < 0.0001). Given the small subgroup size, we combined the eight size/mitotic rate pairings in Table 5 into four groups.
Discussion
GISTs are rare in the esophagus, colon, and appendix, and previous studies of these tumors have been limited to small case series or literature reviews of reported cases. In this study, we identified 265 GISTs arising in these three sites and analyzed the clinicopathologic features that were associated with disease progression.
Esophageal GISTs are rare and account for only 0.7% of all GISTs3. To date, the largest case series in the English literature included only 19 patients11,12,13,14. A recent review of 105 resected esophageal GISTs pooled from the literature published until 2018, including 37 case reports and 8 case series12, revealed a median patient age of 58 years and no significant sex predilection. The reported GISTs were most commonly located in the lower esophagus (72.9%). Most patients presented with tumor-associated symptoms, particularly dysphagia, followed by chest pain and cough. A pure spindle cell morphology was reported in 81% of the cases. The presence of tumor-associated symptoms, tumor necrosis, high mitotic rate, and large tumor size were reportedly associated with worse prognosis, with a disease-specific mortality rate of 19.2% during a median follow-up of 34 months. Our cohort of 102 esophageal GISTs demonstrated a similar demographic distribution and clinical presentation to these 105 pooled cases. Most of our cases were also identified in the distal esophagus, which is explained by the greater abundance of interstitial cells of Cajal in this location15. Crucially, our study also confirms the correlations between tumor size, mitotic count, and presence of symptoms with disease progression. In contrast, disease-specific death was only observed in 2 patients in our cohort, with an overall disease-specific mortality rate of 2%, which is substantially lower than that of these previously reported cases (19.2%). This is likely because many small incidental cases were included in our study (30% of our cases were <1.0 cm). Incidental GISTs of the gastroesophageal junction have been reported to be somewhat common in esophagogastrectomy specimens, but they are usually small and therefore less likely to be published as case reports16. Consistent with this theory, the esophageal cases in our cohort were much smaller than those in the 105-case series (median size of 2.5 cm versus 7.0 cm), and more of them were identified incidentally (50% versus 27.1%).
Colonic GISTs are uncommon, and studies in the literature comprise mostly case reports and small case series, with the largest series including 37 patients17,18. They purportedly account for 2.5–2.9% of all GISTs, according to two recent studies based on Surveillance, Epidemiology, and End Results (SEER) database cases3,19, although it is unclear whether some rectal cases were inadvertently included in this SEER data. The median age of patients with colonic GISTs was reported to be 67.5 years in the most recent SEER database study19, with no significant sex predilection, which is comparable to our cohort. Also consistent with our findings, previous studies documented a slight predilection for the left colon, though left-sided location does not correlate with disease prognosis17,19. Small tumors (<5.0 cm) accounted for 79% of our colonic GISTs, which is a much higher percentage than that reported in the more recent SEER database study (49%)19, potentially indicating reporting bias. Despite this difference, the overall risk of metastasis observed in our study (12%) is comparable to the more recent SEER database study (16%)19. On the other hand, additional case reports and case series have indicated a significantly higher metastasis rate (up to one-third of colonic GISTs), likely because the majority of such reported cases were high-risk18. As in previous studies, we found that tumor size and mitotic rate were strongly associated with disease progression. The presence of symptoms and tumor necrosis were also found to correlate with disease progression. In addition, male patients tended to have a worse prognosis in our study, similar to the findings in reported colonic GIST cases18.
Appendiceal GISTs are extremely rare. Only around 20 cases have been reported in the English literature20,21,22,23,24. The patients in these reported cases had a median age of 67 years and a male:female ratio of 2.4:120. Almost half of the reported patients presented with appendicitis-like symptoms but demonstrated no histologic evidence of appendicitis, suggesting that the symptoms were caused by the tumor20. The tumors were reportedly distributed equally at the base, middle portion, and tip of the appendix. The size of the reported tumors ranged from 0.1 cm to 22.0 cm (median: 1.25 cm). The tumors in five of the reported cases were larger than 2.0 cm (including 3 cases >5.0 cm), and one large tumor (22.0 cm) had a mitotic rate >5/5 mm2 20,25,26. Disease progression (peritoneal metastasis) was reported only in the patient with this large, mitotically active GIST20. In our cohort, 23 of the 27 appendiceal cases were sub-centimeter, and only one tumor was larger than 2.0 cm (measuring 4.5 cm). None of our cases had a mitotic rate above 5/5 mm2, and 22 cases (81%) had no identifiable mitoses. In contrast to the reported cases, only 4 (15%) of our patients presented with lower abdominal pain, 2 of whom had histologic acute appendicitis. Not surprisingly given their small size and low mitotic rate, none of our cases demonstrated disease progression during a median follow-up period of 39 months. These data suggest that the vast majority of appendiceal GISTs are small and mitotically inactive and therefore cured with appendectomy, though the literature indicates that large, mitotically active appendiceal GISTs are capable of progression.
Based largely on several pioneering studies by Miettinen et al.27,28,29,30, it has been widely accepted that tumor size and mitotic rate (per 50 high-power fields [HPF] in their studies, analogous to 5 mm2 on modern microscopes) are the two most powerful criteria for assessment of progression risk in GISTs6. These studies evaluated clinicopathologic features and follow-up data of GISTs arising in the stomach (n = 1552), jejunum/ileum (n = 791), duodenum (n = 156), and rectum (n = 133), and demonstrated that mitotic rate and tumor size are the most significant prognostic factors for GISTs at all of these locations. Furthermore, the studies stratified the tumors at each location into 6–8 groups based on tumor size and mitotic count: tumors with size >5.0 cm and mitotic counts >5/50 HPF have the highest risk of progression, while those with size ≤5.0 cm and mitotic counts ≤5/50 HPF rarely progress. Anatomic location of the primary tumor is also a critical parameter that needs to be considered in risk assessment of GISTs6,31. Although they share similar histomorphologic and molecular features, GISTs arising in different anatomic locations have considerably different biologic behavior. Small bowel, large bowel, and esophageal GISTs are more aggressive than gastric GISTs with similar size and mitotic rate6. Site-specific risk assessment tables exist for gastric, small bowel, and rectal GISTs but not for other uncommon sites of origin. In this multi-institutional study, we used a large cohort of esophageal and colonic GIST cases to generate a table similar to those in widespread use for GISTs in more common locations (Table 5). Although our case numbers are still relatively small, our data indicate that esophageal and colonic GISTs are indeed more likely to progress if the mitotic rate is >5/5 mm2. Tumor size correlates with mitotic counts, as nearly all esophageal and colonic GISTs with mitotic rates >5/5 mm2 were ≥2.0 cm, and most (about 70%) were ≥5.0 cm. Tumors with mitotic rates ≤5/5 mm2 but size > 5.0 cm demonstrated a moderate risk of progression. As with small, low-mitosis GISTs at other sites, esophageal and colonic GISTs ≤5.0 cm with mitotic rates ≤5/5 mm2 were very unlikely to progress. In our cohort, none of the 71 esophageal GISTs or 102 colonic GISTs meeting these two criteria progressed. Our multivariable analysis of the combined esophageal, colonic, and appendiceal GISTs supports the contention that large size and high mitotic rate confer poor prognosis.
Overall, for tumors of similar size and mitotic counts, the biological potential of esophageal and colonic GISTs appears to be roughly between that of gastric and small bowel GISTs. Currently, the CAP10 recommends that GISTs arising in locations without existing risk stratification data be evaluated using jejunum/ileum criteria, and Miettinen and Lasota appear to endorse this approach32. However, based on our data, this practice may slightly overestimate the risk of progression for esophageal and colonic GISTs.
This study has several limitations. First, GISTs at these sites are rare. We included 47 participating institutions, but each institution was only able to provide an average of 5.6 cases. Therefore, our case numbers are still relatively small, particularly with regard to the number of cases that progressed, and the risk of disease progression generated in this study may not reflect the true risk of each tumor risk group, although it likely approximates the true risk. A related issue is that we had fewer than five cases for some size/mitotic rate subgroups, as seen in Supplementary Table 1. However, a similar limitation was also encountered for some pairings in the widely used risk-stratification table for gastric, small bowel, and rectal GISTs. Specifically, some subgroups in this table are combined with an adjacent group to generate an aggregate risk of progression based on small case numbers (which appears to vary by study)6, and two additional non-combined subgroups have a reported risk of progressive disease based on a “small number of cases” (two and eight cases). We employed the same approach when populating our table. Another outcome likely related to the small number of cases is that risk progression does not always increase linearly with increasing size; for instance, among mitotically active esophageal GISTs, those >2.0 to ≤5.0 cm had a 67% risk of progression, compared to 50% for those >5.0 to ≤10.0 cm and 25% for those >10.0 cm. However, this finding is also present, to a lesser degree, in the existing risk-stratification data for rectal GISTs, as mitotically active cases ≤2.0 cm have a slightly higher progression risk (54%) than those >2.0 to ≤5.0 cm (52%)6. Furthermore, those data combined all duodenal and rectal GISTs >5.0 cm into one prognostic calculation for each site and mitotic rate (as above), meaning similar artifacts may have existed prior to the groups being combined.
Second, due to our case numbers, biopsy/polypectomy-only cases (24 colonic and 13 esophageal) were included in this study if dependable radiologic data on size were available. Fortunately, almost all the biopsy/polypectomy-only colonic GISTs (23 of 24 cases) and the majority of the biopsy-only esophageal cases (8 of 13 cases) fit into the lowest risk group (≤2.0 cm and ≤5 mitoses/5 mm2), hence the lack of resection. None of these low-risk colonic and esophageal cases progressed during follow-up. The remaining five biopsy-only esophageal cases had mitotic rate >5/5 mm2 and/or size >5.0 cm, and three of these developed metastasis within 3 months of biopsy. Regardless, there was no significant association between procedure type and disease progression for colonic or esophageal GISTs when stratified by size and mitotic rate (P = 0.18 for esophagus cases and P = 1.0 for colonic cases, with small group sizes). While these cases met our inclusion criteria, risk assessment in this study would remain largely unchanged if these biopsy/polypectomy-only cases were excluded.
Third, some esophageal (n = 15) and colonic (n= 8) GISTs in this study received neoadjuvant imatinib treatment. Most of the treated cases were >5.0 cm in size and/or had a mitotic rate >5/5 mm2. The majority of the esophageal (65%) and colonic (82%) cases with mitotic rate >5/5 mm2 also received adjuvant imatinib treatment. Among the 53 patients who received neoadjuvant and/or adjuvant therapy, 22 (42%) experienced disease progression either before or after treatment. Therefore, the overall inherent biologic risk assessed in this study may be underestimated due to patient treatment, though many treated cases still demonstrated progression. This potential confounder was not a significant factor in the studies by Miettinen et al., which predated imatinib use as standard therapy for advanced GISTs, and affected only four jejunal/ileal cases and two gastric cases in their studies29,30. Since imatinib use has become standard of care for GIST, it is now essentially impossible to accrue a large cohort of high-risk but untreated GISTs.
Fourth, given the rarity of poor outcomes in our series, we could not perform single-site multivariate analyses of risk factors for progression. However, our univariate and combined-site multivariate analyses quite strongly indicate that size and mitotic rate are prognostic indicators for esophageal and colonic GISTs, which was the primary goal of our study. Additionally, multivariate analyses were either not performed or not emphasized in the landmark studies confirming size and mitotic rate as risk factors for GISTs of the stomach, small bowel, and rectum27,28,29,30. Our results suggest that symptomatology and tumor necrosis generally indicate poor prognosis in esophageal and colonic GISTs, though these are quite likely related to tumor size and mitotic rate.
Fifth, median follow-up for the esophageal and colonic GIST patients combined was 34 months, which is relatively short. However, median time to progression in that cohort was 4 months, meaning a median follow-up of 34 months should have adequately captured most progression events.
Finally, given the logistical complexity of including 47 institutions in this study, cases were not submitted for central review. Instead, all cases were evaluated by expert gastrointestinal or soft tissue pathologists at participating institutions. This may have led to some variation in the counting of mitotic rates, as interobserver variability is known to exist in evaluation of this parameter33.
Despite these limitations, we have collected the largest cohort of pathologically reviewed GISTs from the esophagus, colon, and appendix and analyzed the clinicopathologic features associated with disease progression. Our data show that mitotic rate and tumor size are significant prognostic factors for both esophageal and colonic GISTs, as with GISTs arising in more common locations. Furthermore, our results indicate that esophageal, colonic, and appendiceal GISTs with size ≤5.0 cm and mitotic rate ≤5/5 mm2 are unlikely to progress. Other clinicopathologic parameters, including patient sex, symptoms, and tumor necrosis, also appear to correlate well with disease progression for esophageal and colonic GISTs. While GISTs of the esophagus, colon, and appendix are rare, they will be occasionally encountered by clinicians and pathologists, and our data can help inform decisions regarding therapy for these patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kindblom, L. G., Remotti, H. E., Aldenborg, F. & Meis-Kindblom, J. M. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am. J. Pathol. 152, 1259–1269 (1998).
Patel, N. & Benipal, B. Incidence of gastrointestinal stromal tumors in the united states from 2001 to 2015: a United States cancer statistics analysis of 50 states. Cureus 11, e4120 (2019).
Kukar, M. et al. Gastrointestinal stromal tumors (GISTs) at uncommon locations: a large population based analysis. J. Surg. Oncol. 111, 696–701 (2015).
Fletcher, C. D. M. et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum. Pathol. 33, 459–465 (2002).
Miettinen, M., El-Rifai, W., Sobin, L. & Lasota, J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors. Hum. Pathol. 33, 478–483 (2002).
Miettinen, M. & Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 23, 70–83 (2006).
Joensuu, H. et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 13, 265–274 (2012).
Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 39, 1411–1419 (2008).
Gold, J. S. et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 10, 1045–1052 (2009).
Laurini, J. A. et al. Protocol for the examination of resection specimens from patients with gastrointestinal stromal tumor (GIST). GIST Resection 4.1.0.0 2019. https://documents.cap.org/protocols/cp-other-gist-resection-19-4100.pdf.
Robb, W. B. et al. Esophageal gastrointestinal stromal tumor: Is tumoral enucleation a viable therapeutic option? Ann. Surg. 261, 117–124 (2015).
Schizas, D. et al. Prognostic factors affecting mortality in patients with esophageal GISTs. J. BUON. 25, 497–507 (2020).
Pence, K. et al. Management of esophageal gastrointestinal stromal tumor: review of one hundred seven patients. Dis. Esophagus 30, 1–5 (2017).
Lott, S. et al. Gastrointestinal stromal tumors of the esophagus: evaluation of a pooled case series regarding clinicopathological features and clinical outcome. Am. J. Cancer Res. 5, 333–343 (2014).
Radenkovic, G. et al. C-kit-immunopositive interstitial cells of Cajal in human embryonal and fetal oesophagus. Cell Tissue Res. 340, 427–436 (2010).
Abraham, S. C., Krasinskas, A. M., Hofstetter, W. L., Swisher, S. G. & Wu, T. T. “Seedling” mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am. J. Surg. Pathol. 31, 1629–1635 (2007).
Miettinen, M., Sarlomo-Rikala, M., Sobin, L. H. & Lasota, J. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am. J. Surg. Pathol. 24, 1339–1352 (2000).
Feng, F. et al. Clinicopathological features and prognosis of colonic gastrointestinal stromal tumors: evaluation of a pooled case series. Oncotarget 7, 40735–40745 (2016).
Liu, Z. et al. Colonic gastrointestinal stromal tumor: a population-based analysis of incidence and survival. Gastroenterol. Res. Pract. 2019, 3849850 (2019).
Kaneko, M. et al. Giant gastrointestinal stromal tumor of the vermiform appendix: a case report. Mol. Clin. Oncol. 7, 399–403 (2017).
Mourra, N., Matalka, I. & Arrive, L. Incidental finding of gastrointestinal stromal tumor in the appendix. Appl. Immunohistochem. Mol. Morphol. 28, e31–e32 (2020).
Thomsen, T., Ernst, M. & Gottschalk, U. Gastrointestinal stromal tumor of the appendix, extremely rare, mostly benign. Ultraschall. Med. 37, PS6_05 (2016).
Vassos, N. et al. A novel complex KIT mutation in a gastrointestinal stromal tumor of the vermiform appendix. Hum. Pathol. 44, 651–655 (2013).
Lee, M. H., Lee, H. K., Yi, B. H. & Kim, H. K. Gastrointestinal stromal tumor of the appendix mimicking a mucinous cystadenocarcinoma: a case report. J Korean Soc. Radio. 65, 81–83 (2011).
Chung, J. C. & Song, O. P. Gastrointestinal stromal tumor of the appendix. Turk. J. Gastroenterol. 23, 303–304 (2012).
Elazary, R. et al. Malignant appendiceal GIST: case report and review of the literature. J. Gastrointest. Cancer 41, 9–12 (2010).
Miettinen, M. et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum. Am. J. Surg. Pathol. 27, 625–641 (2003).
Miettinen, M. et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am. J. Surg. Pathol. 25, 1121–1133 (2001).
Miettinen, M., Sobin, L. H. & Lasota, J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am. J. Surg. Pathol. 29, 52–68 (2005).
Miettinen, M., Makhlouf, H., Sobin, L. H. & Lasota, J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am. J. Surg. Pathol. 30, 477–489 (2006).
Emory, T. S., Sobin, L. H., Lukes, L., Lee, D. H. & O’Leary, T. J. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am. J. Surg. Pathol. 23, 82–87 (1999).
Miettinen, M. & Lasota, J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab. Med. 130, 1466–1478 (2006).
Agaimy, A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: more questions than answers? A review emphasizing the need for a standardized GIST reporting. Int. J. Clin. Exp. Pathol. 3, 461–471 (2010).
Acknowledgements
We thank Dr. William Jeck of Duke University for his contribution to this study. The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Consortia
Contributions
R.S.G. conceived the study. S.H., L.A., and R.S.G. designed the study and analyzed data. J.M.M.C. provided statistical analysis and expert opinion. S.H. drafted the manuscript. All members of the Rare GIST Risk Stratification Group provided data, reviewed the manuscript, and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The components of the study conducted at each institution were approved by their respective institutional review boards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hu, S., Alpert, L., Cates, J.M.M. et al. Gastrointestinal stromal tumors (GISTs) arising in uncommon locations: clinicopathologic features and risk assessment of esophageal, colonic, and appendiceal GISTs. Mod Pathol 35, 554–563 (2022). https://doi.org/10.1038/s41379-021-00949-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00949-w