Abstract
The 8th Edition of the American Joint Committee on Cancer (AJCC) Staging Manual designates discontinuous involvement of spermatic cord soft tissue by testicular germ cell tumors as a metastatic deposit. We conducted a retrospective international multi-institutional study to validate the current recommendations. Thirty-three (72%) nonseminomatous and 13 (28%) seminomatous testicular germ cell tumors were collected from 15 institutions in America, Europe, and Asia. Testicular tumor size ranged from 1.3 to 18.0 cm (mean: 6.1). Cases were classified as discontinuous involvement of spermatic cord soft tissue (n = 26), continuous cord involvement (n = 17), or cord lymphovascular invasion (n = 3). The mean follow-up was 39 months. Clinical stage for discontinuous involvement of spermatic cord soft-tissue patients was I (local disease) in 2/24 (8%), II (regional disease) in 6/24 (25%), and III (distant disease) in 16/24 (67%) cases; 16 (67%) patients presented with distant metastasis. Clinical stage for continuous cord involvement patients was I in 9/17 (53%), II in 4/17 (23%), and III in 4/17 (23%); 4 (23%) patients presented with distant metastasis. Disease progression was seen in 4 patients with discontinuous involvement of spermatic cord soft tissue and 5 with continuous cord-involvement (p = 0.699). When comparing discontinuous and continuous cord involvement, a significant difference was found in cord margin status (p = 0.044), spermatic cord tumor size (p = 0.016), lymph-node involvement (p = 0.037), distant metastasis (p = 0.010), individual clinical stage (p = 0.003), and nonadvanced vs. advanced disease (p = 0.003) at presentation. In multivariate analysis, after adjusting for age, histology, testicular tumor size, percent of embryonal carcinoma, lymphovascular invasion, and cord margin status, discontinuous involvement of spermatic cord soft tissue was significantly associated (p = 0.011) with advanced clinical stage at presentation. Our findings support the designation of metastatic disease for discontinuous involvement of spermatic cord soft tissue, as introduced by the 8th edition of the AJCC staging.
Similar content being viewed by others
Introduction
Testicular cancer is the most common solid neoplasm of young adults between the ages of 20 and 40 years, however, if detected early, it remains the most treatable solid tumor in this population, with a cure rate of > 70% with current treatment regimens1,2,3.
Testicular germ-cell tumors (TGCT) account for 95% of all testicular tumors4 and are categorized into seminomatous (SGCT) and nonseminomatous (NSGCT)1. A testicular mass suspicious for malignancy requires radical orchiectomy; tumor histology, staging, and serum markers dictate further management3,5.
The staging of testicular cancer is based on disease extent and post-orchiectomy serum tumor marker levels. Based on the American Joint Commission on Cancer (AJCC), tumor-node metastasis (TNM) staging system6, the pathologic stage of a tumor confined to the testis without lymphovascular invasion is pT1; the presence of lymphovascular invasion in the testis or spermatic cord, and/or epididymis or hilar soft-tissue invasion, is categorized as pT2 disease; spermatic cord involvement is equivalent to pathologic stage pT31,6. Lymphovascular invasion and invasion of the spermatic cord remain the main factors associated with increased risk of relapse for TGCT3,7,8,9,10,11,12,13,14,15,16,17.
Clinical staging is utilized as a blueprint for individualized patient prognosis and management and is divided into disease confined to the testis/cord (clinical stage I), disease involving regional (retroperitoneal) lymph nodes (clinical stage II), and distant metastatic disease in non-regional nodes or viscera (clinical stage III)1,6. SGCT is classified as good or intermediate risk based on the absence or presence, respectively, of nonpulmonary visceral metastasis; for NSGCT, higher risk is dictated by markedly elevated post-orchiectomy markers, nonpulmonary visceral metastasis, or primary mediastinal tumor3,18.
Although spermatic cord invasion is currently regarded as pathological stage T319, the 8th Edition of the AJCC Staging Manual distinguishes between two types of invasion: continuous spermatic cord soft-tissue involvement by primary tumor, which is staged as pT3; and discontinuous spermatic cord soft-tissue involvement, which is considered as a metastatic deposit and staged as pM16. To the best of our knowledge, despite trends favoring recurrence in discontinuous vs. continuous spermatic cord involvement20, presently no study has demonstrated a significant difference in clinical outcome between these two patterns of cord involvement.
Herein, we conducted a retrospective, multi-institutional study to further characterize the clinicopathologic features of testicular tumors with spermatic cord involvement to determine if there is a significant difference between discontinuous and continuous spermatic cord involvement to support the upstaging to metastatic disease as introduced by the 8th Edition of the AJCC Staging Manual.
Materials and methods
TGCT with spermatic cord involvement resected between 2005 and 2019 were retrospectively collected from 15 institutions across North and South America, Europe, and Asia. The study were approved by the Institutional Review Boards of the University of Alabama at Birmingham and all other participating institutions. Material-transfer agreements were obtained.
Data were obtained through a data-collecting sheet that included patients’ demographics, clinical and pathologic information, treatment, outcome, and follow-up.
A complete deidentified pathology report with gross and microscopic description, final diagnosis, and selected immunohistochemical (IHC) stains was also obtained. For each case, hematoxylin and eosin and unstained slides or formalin-fixed paraffin-embedded (FFPE) tissue blocks representative of the spermatic cord involvement and of the testicular tumor were received. All slides were reviewed by a genitourinary pathologist (CM-G) without knowledge of the clinical outcome.
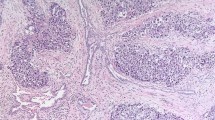
After central review, the cases were classified in three categories: (a) discontinuous involvement of spermatic cord soft tissue (DISC); (b) continuous spermatic cord involvement (CCI); and (c) lymphovascular invasion of spermatic cord vessels (cLVI), in the absence of DISC or CCI. Discontinuous involvement of spermatic cord soft tissue was defined as the presence of a tumor nodule (Fig. 1) or irregular nests or cords of tumor cells in the spermatic cord, separate from the testicular tumor, with the surrounding spermatic cord soft tissue eliciting a desmoplastic stromal reaction. To qualify as discontinuous involvement of spermatic cord soft tissue, tumor cells had to at least involve the soft tissue adjacent to the outermost layer of a vessel wall. CCI was defined as uninterrupted tumor extension from the testis into the spermatic cord, beyond the hilum. Lymphovascular invasion of spermatic cord vessels was defined as cohesive tumor cells confined to a vascular space and adherent to the wall of the vessel.
Immunostains for endothelial cell markers were performed to assess soft-tissue involvement on spermatic cord sections. Stains for CD-31 [(PECAM-1) monoclonal antibody (pre-diluted, WM-59, Biotin, eBioscience™ from Thermo Fisher Scientific), catalog # 13-0319-82, RRID AB_466423] and D2-40 [(Podoplanin) Cell Marque, Ref PA0796, prediluted, Rocklin, CA Keycode CMC00796010] were performed on unstained FFPE sections using an automated BOND-III IHC/ISH platform.
For the purposes of the study, cases were divided into SGCT and NSGCT with further classification based on the percentage of embryonal carcinoma present, since this component is usually the one portraying the worst prognosis. Cases were classified into clinical stage I, II, and III based on available clinical, imaging (chest X-ray and/or computerized tomographic scan), and pathologic information at the time of orchiectomy. Based on clinical and pathologic findings, cases were also classified as M0 (no distant metastasis), M1a (nonretroperitoneal nodal [pelvic, inguinal, or iliac nodes] or pulmonary metastases), and M1b (nonpulmonary visceral metastases). Two patients had biopsy-proven metastatic disease. Disease progression was defined as any additional nodal or visceral disease at follow-up compared with disease burden at presentation; stable disease was defined as no evidence of disease progression at follow-up. The outcome was reported as no evidence of disease (NED), alive with disease (AWD), and dead of disease (DOD) at follow-up.
Statistical analysis
Only DISC and CCI patients were included in the statistical analysis. Categorical variables were summarized with counts and percentages. Statistical analysis was performed using Fisher’s exact test for categorical, Kruskal–Wallis for quantitative variables, and logistic regression for multivariate analysis. The results were considered significant if p < 0.05. Statistical analyses were performed using StataSE 16 (64-bit) and R version 3.6.3 with RSTUDIO Version 1.2.5033. Probability for disease progression between DISC and CCI patients was explored using Kaplan–Meier curve and significance for the estimator was provided using log-rank test. For multivariate analysis, advanced clinical stage (stages II and III) was determined as the endpoint, irrespective of follow-up. Variable treatment modalities were grouped into two categories: chemotherapy alone vs. others (radiation therapy, retroperitoneal lymph-node dissection (RPLND), surveillance, chemotherapy + RPLND, and surveillance + RPLND).
Results
Cohort features
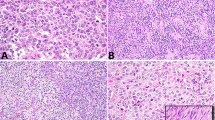
Forty-six TGCT were included in the study. Patient age ranged from 21 to 65 years (mean 38). Thirty-five patients were Caucasian, 4 Hispanic, 2 Asian, 1 Middle-Eastern, 1 African-American, and 3 unknown. Most patients presented with a testicular mass (n = 35), few with pain (n = 4). Twenty-six tumors involved the right and 20 the left testis. Testicular tumor size ranged from 1.3 to 18.0 cm (mean 6.1). Thirty-three (72%) tumors were NSGCT and 13 (28%) SGCT. Twenty-seven (82%) NSGCT harbored embryonal carcinoma, ranging from 10% to 100% of the tumor; 8 cases were pure embryonal carcinoma. Testicular lymphovascular invasion was identified in 41 (95%) cases. After central review, 26 (57%) cases were classified as DISC, 17 (37%) as CCI, and 3 (6%) as cLVI. Lack of staining for CD31 and D2–40 around clusters of tumor cells within the spermatic cord distinguished DISC from cLVI (Fig. 2). Clinical stage at presentation was available for 44 patients; follow-up was available for 30 patients with a mean length of 39 months (range 1–144).
Lymphovascular invasion was present in 23 (88%) of 26 DISC cases. Twenty (77%) DISC tumors were NSGCT: 15 harbored embryonal carcinoma (ranging from 15% to 100% of the tumor), with pure embryonal carcinoma in 6; 2 tumors contained seminoma and yolk sac tumor, 2 tumors contained seminoma, yolk sac tumor, and teratoma; one tumor was composed exclusively of teratoma. Testicular tumors ranged in size from 1.3 to 15.5 cm (mean 5.9) and spermatic cord tumor nodules from 0.3 to 4.5 cm (mean 1.4) (Table 1). Fourteen (56%) DISC tumor nodules involved the proximal spermatic cord, 7 (28%) the mid-spermatic cord, and 4 (16%) the distal spermatic cord; location was unknown in 1 case. The spermatic cord margin of resection was positive in 11 (42%) cases. DISC spermatic tumor nodules consisted of embryonal carcinoma in 13 (50%) cases, seminoma in 8 (30%), yolk sac tumor in 2 (8%) (Fig. 2), teratoma in 2 (8%), and mixed embryonal carcinoma, yolk sac tumor, and teratoma in 1 (4%). Nodal involvement and clinical stage at presentation were available for 24 DISC patients. Fourteen (58%) DISC patients were M1a and 2 (8%) M1b at presentation (Table 1).
Lymphovascular invasion was present in 15 (88%) of 17 CCI cases. Ten (59%) CCI tumors were NSGCT: 9 tumors harbored embryonal carcinoma (ranging from 10% to 100%), with one pure embryonal carcinoma. One CCI case was composed exclusively of yolk sac tumor. Testicular tumor size ranged from 2.8 to 18.0 cm (mean 7.1). The size of the tumor extending beyond the hilum and involving the spermatic cord ranged from 1.2 to 3.0 cm (mean 2.6). The spermatic cord margin of resection was positive in 2 (12%) cases (Table 1). Nodal involvement and CS at presentation were available for all CCI cases. Three (18%) CCI patients were M1a and 1 (6%) M1b at presentation (Table 1).
All 3 cases of cLVI were NSGCT harboring embryonal carcinoma (ranging from 70% to 100% of the tumor). Testicular tumor size ranged from 1.5 to 4.3 cm (mean 3.1). The spermatic cord margin of resection was negative in all cases (Table 1). Nodal involvement and clinical stage at presentation were available for all cLVI cases. One (33%) cLVI patient was M1a at presentation (Table 1).
Patient follow-up, disease progression, and outcome
Follow-up was available for 15 DISC patients and ranged from 4 to 144 months (mean 36.7) (Table 2). Eleven (73%) patients had stable disease at follow-up: 4 presented with regional lymph-node involvement (2 with N1 and 2 with N3) (3 received chemotherapy and 1 radiation), 6 presented with lung metastasis (M1a) (3 underwent chemotherapy and retroperitoneal lymph-node dissection [RPLND], 2 chemotherapy alone, and 1 RPLND), and one presented with lung and nonregional lymph-node involvement (M1b) (treated with chemotherapy and RPLND). Four (27%) DISC patients had disease progression after chemotherapy: one patient developed multifocal visceral disease (M1b) (pulmonary, diaphragm, liver, subcutaneous anterior abdominal wall, and peritoneal nodules); two patients with lung metastasis (M1a) at presentation, subsequently developed regional nodal involvement (N1 and N3, respectively); one patient with regional nodal involvement and lung disease (M1a) at presentation, developed biopsy-confirmed brain metastasis (M1b). Seven DISC patients were NED and 8 AWD at follow-up (Table 2).
Follow-up was available for 14 CCI patients and ranged from one to 122 months (mean 38.9) (Table 2). Nine (64%) patients showed stable disease: 6 had local disease at presentation (clinical stage I) (one patient underwent surveillance, 4 chemotherapy, and 1 radiation); 1 patient presented with regional nodal involvement (clinical stage II) and received chemotherapy; 2 patients presented with visceral metastasis (one with lung involvement [M1a] and 1 with peritoneal, subhepatic, and pelvic involvement [M1b]) and received chemotherapy. The CCI patient with multiple visceral metastases died of disease (DOD) after 5 months. Five (36%) patients had disease progression: of the 3 with local disease (clinical stage I) at presentation, 2 developed regional lymph-node disease (clinical stage II) and 1 developed lung metastasis (M1a); of the 2 patients with regional nodal involvement at presentation (clinical stage II), 1 developed lung metastasis (M1a) and 1 developed biopsy-proven adrenal and mediastinal metastases (M1b). Ten CCI patients were NED and 2 AWD at follow-up (Table 2).
Follow-up was available for 1 of the 3 cLVI patients, who presented with lung metastasis (M1a) and were treated with chemotherapy with stable disease at follow-up (60 months).
Statistical analysis
CLVI cases were excluded from the statistical analysis. When comparing DISC and CCI cases by Fisher’s exact test, there was no difference in patient’s age (p = 0.23), testicular tumor size (p = 0.21), tumor type (SGCT vs. NSGCT) (p = 0.30), percentage of embryonal carcinoma in NSGCT (p = 0.78), lymphovascular invasion (p = 1.00), treatment modality (p = 0.11), and length of follow-up (p = 1.00). Significant difference was found between DISC and CCI in cord margin status (positive vs. negative) (p = 0.044), spermatic cord tumor size (p = 0.016), lymph-node involvement at presentation (N0 vs. N1 vs. N2 vs. N3) (p = 0.037), clinical-stage at presentation (I vs. II vs. III, p = 0.003; I vs. II and III, p = 0.003), and distant metastasis at presentation (M0 vs. M1a vs. M1b) (p = 0.010) (Table 1).
Disease progression vs. stable disease (p = 0.69) was not significantly different between DISC and CCI patients. Kaplan-Meier curve showed no significant difference between CCI and DISC regarding time to disease progression (p = 0.68) (Fig. 3). Of the two CCI patients who died, one died of disease. At follow-up, 7 and 8 DISC and 10 and 2 CCI were NED and AWD, respectively (Table 2). When comparing outcomes, DISC patients fared worse than CCI (p = 0.059), although not significantly.
Using logistic regression, discontinuous involvement of spermatic cord soft tissue was the only variable statistically associated with advanced clinical stage at presentation (p = 0.004) and remained significant in multivariate analysis (p = 0.011), after adjusting for age, testicular tumor size, tumor type (SGCT vs. NSGCT), lymphovascular invasion, percentage of embryonal carcinoma, and cord margin status (Table 3).
Discussion
Pathologic assessment of TGCT is necessary to determine accurate diagnosis, presence of risk factors, and clinical stage, all crucial elements for appropriate treatment selection and patient management, ranging from surveillance to chemotherapy3. The NCCN Guidelines recommend managing patients with clinical stage I NSGCT based on the presence or absence of lymphovascular invasion or invasion of the spermatic cord or the scrotum, factors known to be associated with an increased risk of relapse3,21,22.
Spermatic cord extension of primary testicular tumors has been known as a predictor of recurrence and metastatic potential, particularly in stage-I NSGCT, since the late eighties15,23,24,25. Nazeer et al. reported that “cord involvement increases the likelihood of subsequent metastasis and/or disease recurrence, especially when the tumor is present at the spermatic cord resection margin”15. Rodriguez et al. evaluated the correlation between local extent of primary NSGCT and the presence of metastasis in a cohort of 120 patients and reported that metastasis occurred significantly more frequently in patients with upper or lower spermatic cord involvement26.
The 7th edition of the AJCC staging guidelines defined TGCT with lymphovascular invasion as pT2 and those with spermatic cord involvement, regardless of lymphovascular invasion, as pT327. In 2014, the European network of uro-pathology (ENUP) distributed a survey to its members (n = 225) and experts (n = 25) to assess the evaluation of TGCT and reported significant areas of disagreement in staging28. Twenty-three percent of ENUP members and 28% of experts would have staged a tumor deposit in the upper spermatic cord with a separate testicular tumor associated with vascular invasion as pT2 with a soft-tissue deposit, meanwhile, 75% of ENUP members and 68% of experts would have staged the same lesion as pT3 disease; the remaining participants (2–4%) would have staged the same lesion as a metastasis28. More recently, McCleskey and colleagues evaluated 45 TGCT and found that in patients with NSGCT, lymphovascular invasion in the spermatic cord without soft-tissue invasion is associated with worse clinical stage at presentation compared with patients with lymphovascular invasion limited to the testis29. Some of these studies may have provided evidence to support the changes recently introduced to the pathologic staging based on the type of spermatic cord involvement.
In the AJCC 8th edition, direct invasion of spermatic cord beyond the angle of epididymis and cord is considered as pT3, but it is specified that discontinuous involvement of the spermatic cord and soft tissue via lymphovascular invasion should be considered as a metastatic deposit6. Since the evidence for staging different patterns of spermatic cord, involvement is still limited (level III)30, and there are no convincing data showing differences in clinical outcome between different types of spermatic cord involvement, the current NCCN guidelines recommend, for management purposes, to stage TGCT with discontinuous invasion of the spermatic cord as pT3 and not as pM13.
We conducted a retrospective, multi-institutional study of 46 TGCT to correlate different patterns of spermatic cord involvement with pathologic features, clinical stage, metastatic disease, and disease progression. Seventy-two percent of the cases were NSGCT and 28% SGCT. Twenty-six cases were classified as discontinuous involvement of spermatic cord soft tissues, 17 as continuous or direct involvement of spermatic cord, and three as spermatic cord lymphovascular invasion without soft-tissue invasion. In our cohort, clinical stage at presentation and metastasis at presentation were significantly different in the three groups. However, since only three of the cases included in our study had spermatic cord lymphovascular invasion without soft-tissue invasion, they were excluded from additional statistical analysis.
Lymphovascular invasion in the spermatic cord without soft tissue invasion has been associated with worse clinical stage at presentation when compared with lymphovascular invasion limited to the testis29. Gordetsky and colleagues have reported comparable clinical stage at presentation, disease recurrence, and survival between NSGCT with lymphovascular invasion of spermatic cord without direct soft-tissue involvement (n = 38) and NSGCT with spermatic cord soft-tissue invasion (n = 89), although they did not distinguish between continuous and discontinuous involvement of spermatic cord soft tissue14. When we compared continuous and discontinuous involvement of spermatic cord soft tissue, significant differences were found in cord margin status (p = 0.044), spermatic cord tumor size (p = 0.016), lymph-node involvement at presentation (p = 0.037), clinical stage at presentation (p = 0.003), and distant metastasis at presentation (p = 0.010).
San Francisco et al. have conducted a similar study on a larger cohort of TGCT (n = 100) diagnosed as pT3 and reclassified based on the AJCC 8th edition guidelines into three groups: direct invasion of spermatic cord (pT3, 78%), discontinuous involvement of the spermatic cord stroma (pM1, 18%), or a combination of both (pT3pM1, 4%). Although the authors were unable to identify significant differences between pT3 and pM1 as it pertained to clinical stage at presentation (p = 0.450), and likelihood of recurrence (p = 0.076), they concluded that statistical trends favor that patients with discontinuous involvement of the spermatic cord stroma have higher recurrence than patients with direct invasion of spermatic cord20. In keeping with these data, we found that, after adjusting for patient’s age, tumor type, testicular tumor size, percent of embryonal carcinoma, lymphovascular invasion, and cord margin status, discontinuous involvement of the spermatic cord was significantly associated (p = 0.011) with advanced clinical stage at presentation in multivariate analysis.
When comparing studies, it is worth noting that although mixed TGCT represented the predominant tumor type in both San Francisco’s and the present study (72% vs. 66%), and testicular tumor size (mean 6.1 vs. 6.4 cm) was similar, advanced clinical stage at presentation was more common in San Francisco’s (98%) compared to our (75%) cohort. In contrast to San Francisco’s, our patients with discontinuous involvement of the spermatic cord were more likely to present with advanced disease (92%) compared with patients with continuous/direct involvement of spermatic cord (47%) (p = 0.003). Since the follow-up between the two studies is comparable [range 2–132 months in San Francisco’s vs. 1–144 months in our study], the higher proportion of TGCT with discontinuous involvement of the spermatic cord in our cohort (60%) compared with the San Francisco’s cohort (18%) may explain the discrepancy in results, as supported by a significant Fisher’s exact test between the two cohorts.
In a recent study, Scandura et al.31 analyzed various pathological predictors of metastatic disease in 219 NSGCT and found that on univariate analysis, tumor size, percentage of embryonal carcinoma, lymphovascular invasion, stromal rete testis invasion, hilar soft–tissue invasion, epididymis invasion, direct spermatic cord invasion, and tumor at spermatic cord margin were associated with higher clinical stage. On multivariate analysis, only lymphovascular invasion, tumor size, percentage of embryonal carcinoma, and stroma rete testis invasion remained significant predictor of metastatic disease at presentation. Direct spermatic cord invasion represented only 4% of their cases (n = 10), and most of them (80%) were associated with advanced clinical stage, in contrast to our cohort, where tumors with direct spermatic cord invasion were similarly distributed between patients with advanced (47%, 8/17) and nonadvanced (53%, 9/17) clinical stage.
In the cohort of Scandura et al.31, when embryonal carcinoma was considered a dichotomous variable, no significant differences were identified between clinical stage I and clinical stage II/III, in keeping with other studies9,32. However, the percentage of embryonal carcinoma was a significant predictor of higher clinical stage, and the best-percentage cut off was ≥70%. In the present study, we found no significant differences between discontinuous involvement of spermatic cord soft tissue and CCI cases, neither when considering the presence or absence of embryonal carcinoma or when comparing its percentage; however, it is worth mentioning that embryonal carcinoma represented more than 50% of the tumor in 80% (12/15) of cases of discontinuous involvement of spermatic cord soft tissue and 67% (6/9) of CCI cases.
Necchi et al. presented a novel risk classification for intermediate- and poor-risk NSGCT where they identified four statistically significant risk factors for overall survival: age at the time of diagnosis, brain metastases, lung metastases, and primary mediastinal NSGCT33. The current staging system for testicular cancer distinguishes between pulmonary or nonregional lymph-node involvement (M1a), and nonpulmonary metastatic disease (M1b)6. Using a large, population-based cancer registry, Patel and colleagues evaluated 969 patients with metastatic TGCT, and reported that patients with more than one extrapulmonary metastases exhibited the worst cancer-specific survival vs. isolated pulmonary involvement, and among patients with isolated extrapulmonary involvement, those with brain metastases had the poorest survival, followed by patients with liver and bone metastases34. In our cohort, we found that M1a versus M1b status at presentation was more commonly associated with discontinuous involvement of spermatic cord soft tissue (58% and 8%, respectively) than with CCI (17% and 5%, respectively), with a significant difference (p = 0.010); however, at follow-up, there was no considerable difference between the two groups. One of our CCI patients with multiple visceral metastases (pulmonary, diaphragm, liver, subcutaneous anterior abdominal wall, and peritoneal) died of disease at 5 months. Although patients with discontinuous involvement of spermatic cord soft tissue seemed to have a worse outcome than CCI (p = 0.059), the difference was not statistically significant.
Since we found that discontinuous involvement of spermatic cord was significantly associated with advanced clinical stage at the presentation after adjusting for patients' age, tumor type, tumor size, percent of embryonal carcinoma, lymphovascular invasion, and cord margin status, our findings support its classification as M1 disease in the AJCC 8th edition.
It is worth mentioning that not all national and international datasets, which pathologists refer to in their daily practice, have adopted the same staging system. The International Society of Urological Pathology (ISUP) and the most recent International Collaboration on Cancer Reporting (ICCR) datasets for neoplasia of the testis35 have endorsed the AJCC 8th edition staging system; similarly, the Royal College of Pathologists (RCPath), in their recent datasets for testicular cancers, have made an effort to align with the AJCC 8th edition36; unfortunately, the Union for International Cancer Control (UICC) has not adopted the changes included in the current AJCC37.
Major strengths of our study are its international, multi-institutional nature, the original diagnosis been performed by genitourinary pathologists, and the relatively high number of patients with pathologically confirmed discontinuous involvement of the spermatic cord soft tissue; the limitations include the variable specimen-handling protocols followed at each institution, the fact that only representative slides of each case were available for central review, the limited number of cases to detect some of the recognized predictors of outcome in logistic regression analysis, the higher number of DISC vs. CCI cases, and the lack of follow-up for some of the patients.
In summary, our findings suggest an association between DISC and advanced clinical stage at presentation, supporting the designation of metastatic disease for discontinuous involvement of spermatic cord soft tissue, as introduced by the 8th edition of the AJCC staging.
This study describes extensively the features of DISC and CCI and can be used to design and evaluate more ambitious projects on this subject, necessary to ascertain our conclusion.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ulbright, T., Amin, M. B., Balzer, B., Berney, D. M., Epstein, J. I., Guo, C. et al. Tumours of the testis and paratestitcular tissue. In: Moch H., Humphrey P. A., Ulbright T. M., Reuter V. E. (eds) WHO classification of tumours of the urinary system and male genital organs. IARC press, Lyon. 185–257 (2016).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA. Cancer J. Clin. 70, 7–30 (2020).
Gilligan, T., Lin, D. W., Aggarwal, R., Chism, D., Cost, N. & Derweesh, I. H. et al. Testicular cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 17, 1529–1554 (2019).
Baird, D. C., Meyers, G. J. & Hu, J. S. Testicular cancer: Diagnosis and treatment. Am. Fam. Physician. 97, 261–268 (2018).
Jones, R. H., Vasey, P. A. & Part, I. Testicular cancer–management of early disease. Lancet. Oncol. 4, 730–737 (2003).
Brimo F., Srigley J. R., Ryan C. J., Choyke P. L., Humphrey P., Barocas D. A., et al. Testis. in AJCC cancer staging manual (eds Amin M. B.) 727–735 (Springer 2017).
Tandstad, T., Ståhl, O., Håkansson, U., Dahl, O., Haugnes, H. S. & Klepp, O. H. et al. One course of adjuvant BEP in clinical stage I nonseminoma mature and expanded results from the SWENOTECA group. Ann. Oncol. 25, 2167–2172 (2014).
Ernst, D. S., Brasher, P., Venner, P. M., Czaykowski, P., Moore, M. J. & Reyno, L. et al. Compliance and outcome of patients with stage 1 non-seminomatous germ cell tumors (NSGCT) managed with surveillance programs in seven Canadian centres. Can. J. Urol. 12, 2575–2580 (2005).
Lago-Hernandez, C. A., Feldman, H., O’Donnell, E., Mahal, B. A., Perez, V. & Howard, S. et al. A refined risk stratification scheme for clinical stage 1 NSGCT based on evaluation of both embryonal predominance and lymphovascular invasion. Ann. Oncol. 26, 1396–1401 (2015).
Kobayashi, K., Saito, T., Kitamura, Y., Nobushita, T., Kawasaki, T. & Hara, N. et al. Oncological outcomes in patients with stage I testicular seminoma and nonseminoma: pathological risk factors for relapse and feasibility of surveillance after orchiectomy. Diagn. Pathol. 8, 57 (2013).
Soper, M. S., Hastings, J. R., Cosmatos, H. A., Slezak, J. M., Wang, R. & Lodin, K. Observation versus adjuvant radiation or chemotherapy in the management of stage I seminoma: clinical outcomes and prognostic factors for relapse in a large US cohort. Am. J. Clin. Oncol. 37, 356–359 (2014).
Sturgeon, J. F., Moore, M. J., Kakiashvili, D. M., Duran, I., Anson-Cartwright, L. C. & Berthold, D. R. et al. Non-risk-adapted surveillance in clinical stage I nonseminomatous germ cell tumors: the Princess Margaret Hospital’s experience. Eur. Urol. 59, 556–562 (2011).
Leman, E. S. & Gonzalgo, M. L. Prognostic features and markers for testicular cancer management. Indian. J. Urol. 26, 76–81 (2010).
Gordetsky, J., Sanfrancesco, J., Epstein, J. I., Trevino, K., Xu, H. & Osunkoya, A. et al. Do nonseminomatous germ cell tumors of the testis with lymphovascular invasion of the spermatic cord merit staging as pT3? Am. J. Surg. Pathol. 41, 1397–1402 (2017).
Nazeer, T., Ro, J. Y., Kee, K. H. & Ayala, A. G. Spermatic cord contamination in testicular cancer. Mod. Pathol. 9, 762–766 (1996).
Yilmaz, A., Cheng, T., Zhang, J. & Trpkov, K. Testicular hilum and vascular invasion predict advanced clinical stage in nonseminomatous germ cell tumors. Mod. Pathol. 26, 579–586 (2013).
Blok, J. M., Pluim, I., Daugaard, G., Wagner, T., Jóźwiak, K. & Wilthagen, E. A. et al. Lymphovascular invasion and presence of embryonal carcinoma as risk factors for occult metastatic disease in clinical stage I nonseminomatous germ cell tumour: a systematic review and meta-analysis. BJU. Int. 125, 355–368 (2020).
Wilkinson, P. & Read, G. International germ cell consensus classification: A prognostic factor-based staging system for metastatic germ cell cancers. International germ cell cancer collaborative group. J. Clin. Oncol. 15, 594–603 (1997).
Verrill, C., Yilmaz, A., Srigley, J. R., Amin, M. B., Compérat, E. & Egevad, L. et al. Reporting and staging of testicular germ cell tumors: The international society of urological pathology (ISUP) testicular cancer consultation conference recommendations. Am. J. Surg. Pathol. 41, e22–e32 (2017).
Sanfrancesco, J. M., Trevino, K. E., Xu, H., Ulbright, T. M. & Idrees, M. T. The significance of spermatic cord involvement by testicular germ cell tumors: Should we be staging discontinuous invasion from involved lymphovascular spaces differently from direct extension? Am. J. Surg. Pathol. 42, 306–311 (2018).
Lobo, J., Stoop, H., Gillis, A. J. M., Looijenga, L. H. J. & Oosterhuis, W. Interobserver agreement in vascular invasion scoring and the added value of immunohistochemistry for vascular markers to predict disease relapse in stage I testicular nonseminomas. Am. J. Surg. Pathol. 43, 1711–1719 (2019).
Honecker, F., Aparicio, J., Berney, D., Beyer, J., Bokemeyer, C. & Cathomas, R. et al. ESMO consensus conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann. Oncol. 29, 1658–1686 (2018).
Javadpour, N., Canning, D. A., O’Connell, K. J. & Young, J. D. Predictors of recurrent clinical stage I nonseminomatous testicular cancer. A prospective clinicopathologic study. Urology. 27, 508–511 (1986).
Fung, C. Y., Kalish, L. A., Brodsky, G. L., Richie, J. P. & Garnick, M. B. Stage I nonseminomatous germ cell testicular tumor: prediction of metastatic potential by primary histopathology. J. Clin. Oncol. 6, 1467–1473 (1988).
Javadpour, N. & Young, J. D. Jr Prognostic factors in nonseminomatous testicular cancer. J. Urol. 135, 497–499 (1986).
Rodriguez, P. N., Hafez, G. R. & Messing, E. M. Nonseminomatous germ cell tumor of the testicle: does extensive staging of the primary tumor predict the likelihood of metastatic disease? J. Urol. 136, 604–608 (1986).
Edge S. B., Byrd D. R., Compton C. C., Fritz A. G., Greene F. L., Trotti A. I. AJCC Cancer Staging Manual. 7th edn (Springer 2010).
Berney, D. M., Algaba, F., Amin, M., Delahunt, B., Comperat, E. & Epstein, J. I. et al. Handling and reporting of orchidectomy specimens with testicular cancer: areas of consensus and variation among 25 experts and 225 European pathologists. Histopathology. 67, 313–324 (2015).
McCleskey, B. C., Epstein, J. I., Albany, C., Hashemi-Sadraei, N., Idrees, M. T. & Jorns, J. M. et al. The significance of lymphovascular invasion of the spermatic cord in the absence of cord soft tissue invasion. Arch. Pathol. Lab. Med. 141, 824–829 (2017).
Paner, G. P., Stadler, W. M., Hansel, D. E., Montironi, R., Lin, D. W. & Amin, M. B. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur. Urol. 73, 560–569 (2018).
Scandura, G., Wagner, T., Beltran, L., Alifrangis, C., Shamash, J. & Berney, D. M. Pathological predictors of metastatic disease in testicular non-seminomatous germ cell tumors: which tumor-node-metastasis staging system? Mod. Pathol. 34, 834–841 (2021).
Divrik, R. T., Akdoğan, B., Ozen, H. & Zorlu, F. Outcomes of surveillance protocol of clinical stage I nonseminomatous germ cell tumors-is shift to risk adapted policy justified? J. Urol. 176, 1424–1429 (2006).
Necchi, A., Pond, G. R., Nicolai, N., Giannatempo, P., Raggi, D. & Adra, N. et al. A suggested prognostic reclassification of intermediate and poor-risk nonseminomatous germ cell tumors. Clin. Genitourin. Cancer. 15, 306–312 (2017).
Patel, H. D., Singla, N., Ghandour, R. A., Freifeld, Y., Cheaib, J. G. & Woldu, S. L. et al. Site of extranodal metastasis impacts survival in patients with testicular germ cell tumors. Cancer. 125, 3947–3952 (2019).
Berney, D. M., Comperat, E., Feldman, D. R., Hamilton, R. J., Idrees, M. T. & Samaratunga, H. et al. Datasets for the reporting of neoplasia of the testis: recommendations from the International collaboration on cancer reporting. Histopathology. 74, 171–183 (2019).
Berney D. M., Verrill C. Dataset for histopathological reporting of testicular neoplasms. In: The royal college of pathologists (ed). (London, 2020).
Delahunt, B., Egevad, L., Samaratunga, H., Varma, M., Verrill, C. & Cheville, J. et al. Uicc drops the ball in the 8th edition tnm staging of urological cancers. Histopathology. 71, 5–11 (2017).
Author information
Authors and Affiliations
Contributions
MDCRP, SCP, and CM-G performed study concept and design, data collection, writing, and revision of the paper; CM-G, AA, MA, PC, RC, DEB, IG, FK, KM, RL, HM, AOO, MRR, FS, JS, DLZ, SW, and GJN contributed cases and performed review and editing of the paper; DFS, MDCRP, and SCP performed development of methodology, analysis, and interpretation of data, statistical analysis, and review of the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval/consent to participate
The study was approved by the Institutional Review Boards of the University of Alabama at Birmingham and all other participating institutions. Material transfer agreements were obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodriguez Pena, M.D.C., Canete-Portillo, S., Amin, A. et al. Testicular Germ-Cell Tumors with Spermatic Cord Involvement: A Retrospective International Multi-Institutional Experience. Mod Pathol 35, 249–255 (2022). https://doi.org/10.1038/s41379-021-00912-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00912-9