Abstract
For neoadjuvant therapy in patients with non-small cell lung cancer, the major pathologic response of primary tumors may be an assessable and reliable surrogate measure of survival. Few studies have examined the pathologic evaluation of metastatic lymph node responses and their prognostic significance. This retrospective study enrolled 336 patients with non-small cell lung cancer (squamous cell carcinoma, n = 216; adenocarcinoma, n = 120) treated with neoadjuvant therapy including chemotherapy (n = 316) and targeted therapy (adenocarcinoma, n = 20). The treatment response of the primary tumor and lymph node metastases (LNM) were pathologically assessed according to the multidisciplinary recommendations of the International Association for the Study of Lung Cancer. The relationship of overall survival (OS) and disease-free survival (DFS) with the responses of the primary tumor or LNM was analyzed. The optimal cutoff value of the residual viable tumor (%RVT) of the primary tumor was 12% for both OS (P < 0.001) and DFS (P < 0.001). The pathologic assessment identified LNM in 208 patients. The optimal %RVT cutoff value in LNM was 8% for both OS (P = 0.003) and DFS (P < 0.001). The Spearman’s rank correlation coefficient between primary tumors and corresponding LNM was 0.487 for %RVT (P < 0.001), which indicated a positive correlation. On multivariable analysis, an RVT of the primary tumor ≤12% was an independent prognostic factor for improved OS (P = 0.024), whereas an RVT of LNM ≤ 8% was an independent prognostic factor for increased DFS (P = 0.018). Furthermore, in the neoadjuvant chemotherapy group, the optimal %RVT cutoff values for OS in patients with squamous cell carcinoma and adenocarcinoma in the primary tumor were 12% and 58%, respectively. Considering its convenience and operability in clinical application, a 10% threshold RVT value can be used for prognostic evaluation of LNM and primary tumors of squamous cell carcinoma histology; further studies are needed to confirm the optimal cutoff value for primary tumors of adenocarcinoma.
Similar content being viewed by others
Introduction
After studies showed that neoadjuvant therapy can benefit overall survival (OS), the pathologic assessment after neoadjuvant therapy came into focus [1]. Numerous studies have shown that in patients with locally advanced non-small cell lung cancer, a 10% residual viable tumor (RVT) indicates major pathologic response (MPR) and is ideal for predicting the improvement of long-term prognosis [2,3,4,5,6,7,8,9].
The recent multidisciplinary recommendations for the pathologic assessment of lung cancer resection specimens after neoadjuvant therapy proposed by the International Association for the Study of Lung Cancer include the pathologic evaluation of the treatment response of tumors in lymph nodes or other metastatic sites; however, few studies have conducted the pathologic assessment of metastatic lymph nodes, and its clinical significance remains unclear [10]. In cases with metastasis and primary tumor, which may additionally have intratumoral heterogeneity, variations in the response to neoadjuvant therapy may be observed. The MPR of the primary tumor, called primary tumor-MPR (P-MPR), may not be the same as that of lymph node metastases (LNM), called lymph node metastases-MPR (L-MPR). Furthermore, the responses of LNM to neoadjuvant therapy and their clinical significance remain to be studied.
In this study, we mainly explored the clinical significance of the percentage of RVT in LNM and its relationship with the primary tumor.
Materials and methods
Patients
We retrospectively reviewed the pathology database of patients with stage II-III non-small cell lung cancer, squamous cell carcinoma (SCC), and adenocarcinoma (ADC), treated at the Peking University Cancer Hospital between January 2009 and March 2019. We included patients who underwent neoadjuvant therapy, including neoadjuvant chemotherapy and neoadjuvant targeted therapy, followed by surgical tumor resection, i.e., lobectomy or pneumonectomy. Chest computed tomography (CT) examination was performed using a helical CT scanner (Lightspeed VCT, GE Healthcare, Milwaukee, WI). The CT response was assessed using Response Evaluation Criteria in Solid Tumors version 1.1 by an experienced radiologist (M.L.C). The follow-up started from the day of surgery (median follow-up time: 29.0 months, average follow-up time: 34.5 months). Patients with short-term mortality (i.e., 30 days) that could be assigned to postsurgical complications were excluded from the study. From the patient medical records, we obtained detailed clinical data for all patients in the study group, including demographic data, clinical and pathological tumor-node-metastasis staging, OS, disease-free survival (DFS), and other meaningful information.
Histopathologic assessments
Specimens from the surgically resected tumor were routinely fixed in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin. Tumors with a maximum diameter of <3 cm, or cases suspected of no remaining tumors were entirely sampled. All lymph nodes were completely embedded; lymph nodes with a diameter of >5 mm were bisected and those with a diameter of ≤5 mm were sampled directly. Two experienced senior pathologists (X.Y.L. and W.S.) reviewed all the pathological sections and independently determined the percentage of RVT without being informed of the patients’ survival prognosis. An Olympus BX41 microscope with a standard 22-mm diameter eyepiece was used to review the pathological slides. For cases with diverging evaluations, a multi-head microscope was used to obtain the final results by consensus. The surgical specimens of primary lung tumors and lymph nodes were staged according to the criteria of the American Joint Committee on Cancer (8th edition) to evaluate tumor size, affected lymph nodes, and metastases. Tumors were classified according to the 2015 WHO classification [11, 12].
According to the International Association for the Study of Lung Cancer multidisciplinary recommendations, the tumor bed was defined as the area where the original tumor (before neoadjuvant therapy) was considered to be located, comprising three major components: necrosis, stromal fibrosis, and viable tumor. Reactive changes in the surrounding non-neoplastic lung were not considered as parts of the tumor bed [10]. For lymph nodes without viable tumor cells, histological characteristics such as mucus pools without tumor cells, foam cell infiltration, formation of cholesterol fissure crystals, and a combination of radiologic changes and preoperative biopsies were used to help identify the tumor bed. The percentages of necrosis, stromal fibrosis, and viable tumor were estimated based on the review of the microscopic sections on each slide, and the total percentage of viable tumor was estimated. The three components were estimated in 5% increments and totaled 100% of the tumor bed.
Statistical analysis
The associations between clinical and pathological features and histologic types were analyzed using the chi-square test or Fisher’s exact test (for categorical variables) and the Wilcoxon rank-sum test (for continuous variables). Correlations were evaluated using Spearman’s rank correlation coefficient. Consistency between observers was evaluated by randomly sampling 10% of all cases, and the results were analyzed using intraclass correlation coefficients and Bland–Altman plots. OS was calculated as the time from surgery to death owing to any cause, and DFS was calculated as the time from the surgery to recurrence, metastasis, or last follow-up. The optimal cutoff percentages of histologic components for predicting OS and DFS were estimated using the maximally selected log-rank statistic with the R package maxstat [9]. Survival probability as a function of time was computed using the Kaplan–Meier method. The log-rank test was used to compare OS and DFS between groups. Univariable Cox proportional hazards regression analysis was used to examine the association between various prognostic factors and OS or DFS. Variables with p-values of <0.05 in the univariable analysis were then evaluated by multivariable analysis using the Cox proportional hazards regression model. All analyses were performed using IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA) and R version 4.0.3 (The R Project for Statistical Computing, Vienna, Austria). Two-sided p-values of <0.05 were considered statistically significant.
Results
Clinical and histopathological features
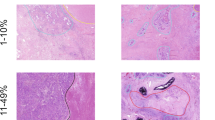
A total of 336 patients were enrolled in this study. In this study population, 266 and 70 patients were men and women, respectively, with a median age of 59 years (interquartile range: 54–64 years). SCC was diagnosed in 216 cases, and all of these patients received neoadjuvant chemotherapy. Among the 120 patients with ADC, 100 received neoadjuvant chemotherapy, and 20 received neoadjuvant targeted therapy. The number of slides submitted per cm of tumor is shown in Supplementary Fig. S1. The median number of dissected lymph nodes was 22 (range, 3–84) (Supplementary Fig. S2). The general clinicopathological characteristics of all patients are shown in Table 1. The histopathological features of the treatment for metastatic lymph nodes and primary tumors are presented in Fig. 1.
A Necrosis and foam cell infiltration in a metastatic lymph node achieving pathologic complete response without residual tumor cells. B Fibrosis and foam cell infiltration in a metastatic lymph node achieving pathologic complete response without residual tumor cells and necrosis. C Focal squamous cell carcinoma (arrow) remains in a metastatic lymph node achieving lymph node metastases-major pathologic response. D Calcification and cholesterol cleft in a metastatic lymph node with numerous residual viable cells. E A large number of squamous cell carcinoma remains in a metastatic lymph node with almost no therapy treatment response. F Tumor bed including residual viable tumor and large necrosis is surrounded by organizing pneumonia.
Interobserver reproducibility of %RVT scoring
The assessments by the pathologists had a high degree of interobserver reliability for both the primary tumor (Intraclass correlation=0.994; 95% confidence interval [CI]: 0.989–0.997) and LNM (Intraclass correlation=0.998; 95% CI: 0.996–0.999). Based on the Bland–Altman plot, the differences were all close to 0, indicating the absence of systematic bias (Supplementary Fig. S3).
Clinical significance of the 10% and 65% cutoff values
The traditional MPR (≤10% RVT) in the overall primary tumor cohort was associated with better OS (P < 0.001) and DFS (P = 0.001); that of LNM was also associated with better OS (P = 0.003) and DFS (P < 0.001) (Supplementary Fig. S4).
In the chemotherapy group, for patients with SCC, an RVT of ≤10% in the primary tumor was associated with better OS (P = 0.001) and DFS (P = 0.006); for LNM, it was associated with better DFS (P = 0.002). As for patients with ADC, for both the primary tumor and LNM, there was no statistically significant difference between ≤65% RVT and OS (primary tumor: P = 0.087; LNM: P = 0.071) or DFS (primary tumor: P = 0.125; LNM: P = 0.360) (Supplementary Fig. S5).
Optimal %RVT cutoff value for OS and DFS in primary tumors
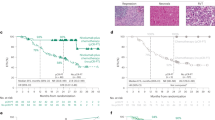
The optimal cutoff percentages of RVT for predicting OS and DFS were determined separately using maximally selected log-rank statistics. The best %RVT cutoff values for OS and DFS were both 12% (Supplementary Fig. S6A and S6B). Figure 2A shows the 3-year and 5-year OS for patients by RVT percentage of the primary tumor.
A Overall cumulative mortality by %RVT of primary tumor is shown. The blue and red curves represent 3-year and 5-year overall cumulative mortality respectively. B Kaplan–Meier estimates of overall survival (OS) based on %RVT of PT is shown. Patients with high RVT had significantly worse OS than those with low RVT. C Kaplan–Meier estimates of disease-free survival (DFS) based on %RVT of PT is shown. Patients with high RVT had significantly worse DFS than those with low RVT. D Overall cumulative recurrence rate by %RVT of LNM is shown. The blue and red curves represent 2-year and 3-year overall cumulative mortality, respectively. E Kaplan–Meier estimates of OS based on %RVT of LNM is shown. Patients with high RVT had significantly worse OS than those with low RVT. F Kaplan–Meier estimates of DFS based on %RVT of LNM is shown. Patients with high RVT had significantly worse DFS than those with low RVT.
A total of 87 cases (25.9%) had no more than 10% RVT and achieved traditional MPR, whereas 93 cases (27.7%) had an RVT ≤ 12% and achieved P-MPR. For RVT values of ≤12%, the number of patients with squamous cell carcinoma and adenocarcinoma were 36.6% (79/216) and 11.7% (14/120), respectively. Thus, only six cases (1.8%) had an RVT between 10 and 12%. The OS and DFS curves for patients with and without P-MPR in the overall cohort are shown in Fig. 2. P-MPR was associated with significantly better OS and DFS (P-MPR versus no P-MPR: 5-year OS, 91.3% versus 54.4%, P < 0.001; 3-year DFS, 74.7% versus 48.1%, P < 0.001; Fig. 2B and C). The cumulative hazard values for death and recurrence for patients with low (≤12%) and high (>12%) RVT in primary tumor are shown in Supplementary Fig. S7A and S7B.
In the neoadjuvant chemotherapy group, the optimal %RVT cutoff values for OS in patients with SCC was 12%, whereas in patients with ADC the cutoff value for OS was 58%. Supplementary Fig. S8 shows OS curves comparing patients with low RVT (>optimal cutoff) with patients with high RVT (≤optimal cutoff) in the SCC and ADC cohorts.
Optimal %RVT cutoff value for OS and DFS in LNM
In this study, 208 cases were positive for lymph node metastasis or had pathological changes related to treatment with no tumor cells remaining. The optimal RVT percentage cutoff values for predicting OS and DFS in patients with LNM were analyzed using maximally selected log-rank statistics. The results showed that the best cutoff values for OS and DFS were both 8% (Supplementary Fig. S6C and S6D). For patients with LNM, the 2-year and 3-year DFS values by %RVT of metastatic lymph nodes are shown in Fig. 2D.
There were 73 cases (35.1%) achieving L-MPR. The L-MPR values in SCC and ADC were 41.2% (54/131) and 24.7% (19/77), respectively. In the pathologic assessment, 29.8% cases (62/208) had a complete lymph node response. Patients with low (≤8%) RVT had significantly better OS and DFS than those with high RVT (low versus high RVT: 5-year OS, 85.1% versus 51.8%, P = 0.003; 3-year DFS, 65.8% versus 36.7%, P < 0.001; Fig. 2E and F). Cumulative hazard values for death and recurrence for patients with low (≤8%) and high (>8%) RVT in LNM are shown in Supplementary Fig. S7C and S7D.
Univariable and multivariable analyses of OS and DFS in primary tumors and LNM
Table 2 shows the results of the univariable Cox proportional hazards regression analyses for OS and DFS based on %RVT of the primary tumor and LNM. In the univariable analysis, pathologic nodal status, lymphovascular invasion, RVT of primary tumors ≤12%, and RVT of LNM ≤ 8% were significantly associated with both OS and DFS. Moreover, the primary tumor stage and pleural invasion were also associated with DFS. In the multivariable model, an %RVT of primary tumors of >12% was associated with lower OS (hazard ratio [HR]: 3.382; 95% CI: 1.175–9.732; P = 0.024), independent of lymphovascular invasion and %RVT of LNM. However, an LNM %RVT of >8% was associated with lower DFS (HR: 1.956; 95% CI: 1.124–3.406; P = 0.018), independent of the primary tumor stage, pleural invasion, lymphovascular invasion and %RVT of primary tumors (Table 3). Due to the strong association of RVT with LNM, the pathologic nodal status was not included in the multivariable regression model.
RVT relationship between primary tumor and corresponding LNM
In LNM-positive cases, the pathologic evaluation of LNM was also compared with that of the corresponding primary tumor lesion. The differences in %RVT value represented a normal distribution. Most cases were in the range of 0% to ±10% (n = 68, 32.7%; Fig. 3A and B). The Spearman’s rank correlation coefficient between primary tumors and corresponding LNM was 0.487 for the percentage of RVT (P < 0.001; Fig. 3C).
Comparison between P-MPR and L-MPR are shown in Table 4. The preoperative and postoperative N-staging in 128 cases was N0; pathological evaluation of the lymph node response was therefore not performed in these cases. Among the 93 cases that achieved P-MPR (≤12% RVT), 52 cases had LNM, and in 36 cases (69.2%), the RVT of LNM was ≤8%. Among the 73 cases that achieved L-MPR, 36 achieved P-MPR (49.3%). Among the 23 cases (11.1%) with LNM and nearly 100% of residual tumor indicating almost no response to neoadjuvant therapy, in one case RVT of the primary tumor was <1%; in another, it was 17%, and the remaining 21 cases had an RVT of >40%. According to the follow-up information for the case with a primary tumor RVT of <1%, this patient is alive but experienced new lymph node metastases.
Comparison between radiologic and pathologic LNM evaluations
Before the neoadjuvant treatment, 253 cases with a clinical N1–N3 stage were detected by CT. Among them, 182 cases (71.9%) presented with metastases or treatment response-related pathological changes in lymph node specimens after surgery. Among the remaining 71 patients without tumor metastasis or significant changes in response to treatment, a large number presented signs of carbon dust (90.1%, 64/71), slight fibrosis (36.6%, 26/71), and mild vasodilation with or without congestion (12.7%, 9/71). The sensitivity and specificity of the diagnosis of LNM by radiologic modalities were 87.5% (182/208) and 44.5% (57/128), respectively.
Discussion
Junker et al. first proposed the grading of regression in non-small cell lung cancer after neoadjuvant therapy in 2001, and found that a therapy-induced tumor regression of <10% of the vital tumor tissue is pivotal for superior long-term outcomes [3]. In 2014, a metric of ≤10% residual tumor tissue, called “major pathologic response, MPR,” which could be used as a surrogate endpoint for neoadjuvant therapy, was termed by Hellmann et al [7]. Based on the 10% cutoff value determined in previous studies, many retrospective studies have verified the predictive significance of MPR and showed that the percentage of cases achieving MPR ranged from 16% to 37% in neoadjuvant chemotherapy [4, 5, 9, 13,14,15,16,17,18,19]. Our data were statistically significant for a cutoff value of 12%, which almost matches the definition of MPR previously established in the literature, and the percentage of cases that achieved P-MPR was 27.7%. The data from this group showed that the optimal cutoff values for SCC and ADC were 12% and 58%, respectively, which were very close to the values of 10% and 65% reported in previous studies [9, 20]. Although the results were not consistent, the overall trend was consistent, indicating that the best cutoff values of %RVT for which SCC and ADC could predict survival were not the same: they were much higher for ADC than for SCC. Further studies are required to confirm the optimal cutoff value for primary tumors of ADC. There were 6 cases with %RVT values greater than 10% and less than or equal to 12% for primary tumor. The difference in the number of cases was not significant for either primary tumors or metastases, and therefore, had no effect on the results. Accordingly, we recommend the use of 10% values for primary tumors of SCC in clinical practice. Using univariable Cox proportional hazards regression analysis for OS and DFS, pathologic nodal status, lymphovascular invasion, and an RVT of ≤12% in primary tumors were significantly associated with both OS and DFS, whereas the primary tumor stage and pleural invasion were additionally associated with DFS. However, the multivariable analysis revealed that a primary tumor RVT of no more than 12% was an independent prognostic factor for improved OS.
Currently, MPR is mainly aimed at the RVT assessment of primary tumors and does not include the LNM assessment. Studies have shown that downstaging of mediastinal lymph nodes metastases after neoadjuvant therapy is related to prognosis [21]. Patients with post-therapeutic N0 or N1 lymph node status were found to have a higher survival benefit than patients with N2 lymph node involvement [22]. Although lymph node involvement is generally one of the most important prognostic factors in lung cancer, there are few relevant studies on the assessment of LNM after neoadjuvant therapy. It is necessary to explore the pathologic evaluation of LNM to better understand its clinical significance. One study suggested that after neoadjuvant therapy, the percentages of therapy-induced necrosis and still vital tumor tissue in dissected lymph nodes should be microscopically determined. Their regression grading system included therapy-induced effects on the primary tumor, as well as on regional lymph nodes [23]. However, they did not separately assess the responses of the primary tumor and lymph nodes, and the evaluation method was not standardized, providing only a rough estimation rather than a precise calculation. Since this recommendation, there have been few studies evaluating the efficacy of lymph node response assessments, and there are currently no complete data reports showing their significance. The LNM assessment after neoadjuvant therapy stagnated for a long time with no substantial progress. Recently, the International Association for the Study of Lung Cancer proposed multidisciplinary recommendations for the pathologic assessment of lung cancer resection specimens after neoadjuvant therapy, which included the assessment of LNM [10]. With reference to these recommendations, our study tried to accurately assess the treatment effects on LNM and intended to determine the %RVT cutoff that has a prognostic value. Our statistical analyses revealed an optimal %RVT cutoff value of 8% in LNM specimens that was related to the patients’ prognosis, especially to DFS, in the multivariable analysis. This indicates that the pathologic evaluation of lymph nodes is necessary and is as important as that of the primary tumor. There were only 3 cases with %RVT values greater than 8% and less than or equal to 10% for LNM. Considering the practical aspects of a routine clinical workup, we recommend the use of a RVT value of 10% for the evaluation of LNM.
Although there may be some differences in terms of treatment responses between primary tumors and LNM, the results were fairly consistent. Our study showed that the absolute value of the difference in %RVT was within 10% in nearly one-third of cases (33%, 68/208). To our knowledge, this is the first study to report the relationship between RVTs of primary tumors and their corresponding LNM specifically. Among the 23 cases with LNM RVT values close to 100%, one had an RVT of less than 1% in the primary tumor. According to the follow-up of this patient, his overall survival had reached 46.5 months, but hilar lymph node metastases were detected at 26 months. This result further demonstrated that the RVT of the primary tumor was closely related to the long-term prognosis, whereas the RVT of LNM was related to short-term recurrence and metastasis. Based on the rare but non-negligible inconsistencies in the responses of the primary tumors and their metastatic lesions to neoadjuvant therapy, a comprehensive pathologic evaluation of both should be required for a precise prognostic assessment. Although the assessment of the primary tumor is prognostically meaningful, the assessment of the lymph nodes is equally important and significant.
In clinical practice, some patients with non-small cell lung cancer might undergo excessive staging because of enlarged lymph nodes detected on CT, particularly when combined with PET, leading to the initiation neoadjuvant therapy [14, 24, 25], but after the treatment, the pathologic evaluation of the lymph nodes might reveal neither residual tumors nor evidence of treatment responses. In our data, 71 cases were considered to be positive for LNM in the preoperative evaluation, but microscopically with no residual tumors or clear evidence of treatment response. According to Wilkinson and Hause, the failure to identify a tumor within a lymph node is related to the size of the lymph node, among other factors [26]. Although lymph nodes larger than 1 cm in diameter were considered abnormal on CT, studies have shown that size is not a reliable predictor of tumor involvement [27]. In 48 cases (67.6%, 48/71), the maximum diameter of the lymph node exceeded 1 cm, which might be considered as a metastatic lymph node in clinical and radiologic assessments. More than 70% of carbon dust depositions could be observed in more than 90% of the enlarged lymph nodes. Almost 40% of cases with slight fibrosis showed no treatment-associated response, which may mislead the physician into doubt the radiologic-based diagnosis of a metastatic process. Our results may help clinicians and radiologists improve the accuracy of the radiologic diagnosis of LNM. Reliable results can be obtained by combining clinical information, radiologic manifestations, and pathological biopsy findings.
The following limitations should be considered: first, this was a retrospective study, so some constraints were inevitable. The targeted therapy group was small and the ADC group was relatively small. Another constraint was that we could only review the slides that had already been taken. The second limitation is with regards to the endpoint of the study. Since some cases underwent neoadjuvant therapy within the past three years, the follow-up times of these cases were not long enough, and more accurate OS results need further follow-up data for confirmation. Third, it was difficult to identify the lymph node tumor bed, especially in cases with fibrosis. These limitations suggest that further research is necessary to improve the existing methodology.
In summary, the percentages of RVT are correlated between the primary tumor and LNM, although there are inconsistencies in a few cases. Clinically, the %RVT cutoff value of 8% for lymph nodes has prognostic significance, especially regarding DFS. In primary tumors, an %RVT of ≤12% was mainly related to OS. It is recommended that an %RVT value of 10% is used in clinical practice for LNM and primary tumors of SCC; further studies are required to confirm the optimal cutoff value for primary tumors of ADC.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Blumenthal GM, Bunn PA Jr, Chaft JE, McCoach CE, Perez EA, Scagliotti GV, et al. Current status and future perspectives on neoadjuvant therapy in lung cancer. J Thorac Oncol. 2018;13:1818–31.
Junker K, Thomas M, Schulmann K, Klinke F, Bosse U, Müller K-M. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological Assess J Cancer Res. Clin Oncol. 1997;123:469–77.
Junker K, Langner K, Klinke F, Bosse U, Thomas M. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest. 2001;120:1584–91.
Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, Kim ES, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2012;7:825–32.
Chaft JE, Rusch V, Ginsberg MS, Paik PK, Finley DJ, Kris MG, et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous non-small-cell lung cancers. J Thorac Oncol. 2013;8:1084–90.
William WN Jr, Pataer A, Kalhor N, Correa AM, Rice DC, Wistuba II, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2013;8:222–8.
Hellmann MD, Chaft JE, William WN, Rusch V, Pisters KMW, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15:e42–e50.
Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–86.
Qu Y, Emoto K, Eguchi T, Aly RG, Zheng H, Chaft JE, et al. Pathologic assessment after neoadjuvant chemotherapy for NSCLC: importance and implications of distinguishing adenocarcinoma from squamous cell carcinoma. J Thorac Oncol. 2019;14:482–93.
Travis WD, Dacic S, Wistuba I, Sholl L, Adusumilli P, Bubendorf L, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15:709–40.
Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:138–55.
Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, et al. The IASLC lung cancer staging project: proposals for coding t categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the tnm classification of lung cancer. J Thorac Oncol. 2016;11:1204–23.
Betticher DC, Hsu Schmitz SF, Totsch M, Hansen E, Joss C, von Briel C, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer. 2006;94:1099–106.
Lee HY, Lee HJ, Kim YT, Kang CH, Jang BG, Chung DH, et al. Value of combined interpretation of computed tomography response and positron emission tomography response for prediction of prognosis after neoadjuvant chemotherapy in non-small cell lung cancer. J Thorac Oncol. 2010;5:497–503.
Li J, Li ZN, Yu LC, Bao QL, Wu JR, Shi SB, et al. Association of expression of MRP1, BCRP, LRP and ERCC1 with outcome of patients with locally advanced non-small cell lung cancer who received neoadjuvant chemotherapy. Lung Cancer. 2010;69:116–22.
Stefani A, Alifano M, Bobbio A, Grigoroiu M, Jouni R, Magdeleinat P, et al. Which patients should be operated on after induction chemotherapy for N2 non-small cell lung cancer? Analysis of a 7-year experience in 175 patients. J Thorac Cardiovasc Surg. 2010;140:356–63.
Mouillet G, Monnet E, Milleron B, Puyraveau M, Quoix E, David P, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol. 2012;7:841–9.
Liao WY, Chen JH, Wu M, Shih JY, Chen KY, Ho CC, et al. Neoadjuvant chemotherapy with docetaxel-cisplatin in patients with stage III N2 non-small-cell lung cancer. Clin Lung Cancer. 2013;14:418–24.
Remark R, Lupo A, Alifano M, Biton J, Ouakrim H, Stefani A, et al. Immune contexture and histological response after neoadjuvant chemotherapy predict clinical outcome of lung cancer patients. Oncoimmunology. 2016;5:e1255394.
Zens, P, Bello, C, Scherz, A, Koenigsdorf, J, Pollinger, A, Schmid, RA et al. A prognostic score for non-small cell lung cancer resected after neoadjuvant therapy in comparison with the tumor-node-metastases classification and major pathological response. Mod Pathol. https://doi.org/10.1038/s41379-021-00777-y (2021).
Choi NC, Carey RW, Daly W, Mathisen D, Wain J, Wright C, et al. Potential impact on survival of improved tumor downstaging and resection rate by preoperative twice-daily radiation and concurrent chemotherapy in stage IIIA non-small-cell lung cancer. J Clin Oncol. 1997;15:712–22.
Langner K, Thomas M, Klinke F, Bosse U, Heinecke A, Muller KM, et al. [Neoadjuvant therapy in non-small cell lung cancer. Prognostic impact of “mediastinal downstaging”]. Chirurg. 2003;74:42–48. discussion 49
Junker K. Histopathologic evaluation of mediastinal lymph nodes in lung cancer. Lung Cancer. 2004;45:S79–S83.
Prenzel KL, Monig SP, Sinning JM, Baldus SE, Brochhagen HG, Schneider PM, et al. Lymph node size and metastatic infiltration in non-small cell lung cancer. Chest. 2003;123:463–7.
Al-Sarraf N, Gately K, Lucey J, Wilson L, McGovern E, Young V. Lymph node staging by means of positron emission tomography is less accurate in non-small cell lung cancer patients with enlarged lymph nodes: analysis of 1,145 lymph nodes. Lung Cancer. 2008;60:62–68.
Wilkinson EJ, Hause L. Probability in lymph node sectioning. Cancer. 1974;33:1269–74.
Tieu BH, Sanborn RE, Thomas CR Jr. Neoadjuvant therapy for resectable non-small cell lung cancer with mediastinal lymph node involvement. Thorac Surg Clin. 2008;18:403–15.
Acknowledgements
We thank Ziming Wang for his contribution in providing data analysis.
Funding
This work was supported by the National Natural Science Foundation of China (81871860 and 82003155), the Capital’s Funds for Health Improvement and Research (2020-2-1025).
Author information
Authors and Affiliations
Contributions
X.Y.L. and D.M.L. performed study concept and design; X.Y.L., W.S., J.H.W., and D.M.L. performed development of methodology and writing, review and revision of the paper; X.Y.L., W.S., J.H.W., L.N.M., and D.M.L. provided acquisition, analysis and interpretation of data, and statistical analysis; M.L.C., Y.F., and Y.Y. provided technical and material support; X.Y., H.Y.W., K.W.C. provided support for revisions. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
This study was approved by the Ethics Committee of Peking University Cancer Hospital (2018YJZ19).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, X., Sun, W., Wu, J. et al. Major pathologic response assessment and clinical significance of metastatic lymph nodes after neoadjuvant therapy for non-small cell lung cancer. Mod Pathol 34, 1990–1998 (2021). https://doi.org/10.1038/s41379-021-00871-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00871-1
This article is cited by
-
Pathological response and tumor stroma immunogenic features predict long-term survival in non-small cell lung cancer after neoadjuvant chemotherapy
Cellular Oncology (2024)
-
“Major pathologic response” in lymph nodes: a modified nodal classification for non-small cell lung cancer patients treated with neoadjuvant immunochemotherapy
Experimental Hematology & Oncology (2023)
-
Clinical utility of [18F]FDG PET/CT in the assessment of mediastinal lymph node disease after neoadjuvant chemoimmunotherapy for non-small cell lung cancer
European Radiology (2023)