Abstract
Vibration-controlled transient elastography (VCTE) is a non-invasive method of evaluating liver fibrosis and steatosis. It can easily be performed in the outpatient setting and has been suggested as an alternative to liver biopsy. However, VCTE and biopsy discrepancies commonly occur. Patient characteristics, procedure performance, and liver features can impact the reliability of VCTE results. We identified 82 patients who received VCTE and biopsy within one month to assess how frequently major discrepancies occur and to determine the role of the liver biopsy in this workup. In our study, 35.4% of patients had a major fibrosis discrepancy, which was defined as advanced fibrosis or cirrhosis by VCTE and no to minimal fibrosis on biopsy. This was significantly associated with increased BMI, and liver features including steatohepatitis, inflammation, congestion, and cholestasis were important contributors to discrepancies. All patients with advanced fibrosis or cirrhosis on liver biopsy were appropriately detected by VCTE (n = 28). Detection of steatosis was less sensitive as 19% (n = 4 of 21) of patients with moderate to severe steatosis on biopsy were missed by VCTE. Liver biopsy has been traditionally performed for diagnosis, but with the emergence of non-invasive tools to evaluate for liver fibrosis and steatosis, biopsies are now additionally being performed to confirm findings from noninvasive procedures. Although VCTE is a highly sensitive tool for liver fibrosis, it is not as specific, and therefore, the liver biopsy remains the gold standard for accurate fibrosis assessment.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD), which affects up to 25% of the world population, refers to the abnormal accumulation of lipids within hepatocytes [1,2,3]. NAFLD can develop into nonalcoholic steatohepatitis (NASH) [4], which is associated with increased risk for diabetes, heart disease, renal disease, and cancer. A subset of cases further progress to severe hepatic fibrosis and cirrhosis, as well as hepatocellular carcinoma [3]. Liver fibrosis is the main predictor of poor outcome, and NASH is currently the leading cause of cirrhosis and indication for liver transplantation in the United States [2].
Approximately 25% of NAFLD cases eventually progress to NASH; risk factors for progression include metabolic syndrome, morbid obesity (BMI > 35), diabetes, dyslipidemia, hypertension, and Hispanic ethnicity [3, 5]. However, these risk factors do not reliably predict who will develop NASH, and identifying high-risk patients presents a major challenge. Currently, there are no consensus guidelines for NALFD or NASH screening in the United States [2]. Studies that screened patients for NAFLD have shown that up to 47% of patients may not be obese and up to 79% of patients may have normal liver function tests, exemplifying the difficulties in formulating a screening method [6, 7]. Liver biopsy is the gold standard for staging fibrosis, but it is an invasive procedure with sample-related limitations [3]. Therefore, there is a demand for non-invasive tests to routinely evaluate steatosis and stage fibrosis in NAFLD and NASH patients. Over the last decade, vibration-controlled transient elastography (VCTE, FibroScan, Echosens, France) has gained traction as a sensitive, noninvasive tool to screen for liver steatosis and advanced fibrosis [3].
VCTE uses a special probe to generate low amplitude and low-frequency vibrations [2]. By Hooke’s law, the velocity of the shear wave through the liver can be converted into a liver stiffness measurement (LSM) that estimates the degree of fibrosis [2]. The fibrosis score is measured in kilopascals (kPa) ranging from 1 to 75 kPa, with the normal range being 3.3–7.8 kPa [8]. VCTE also provides a controlled attenuation parameter (CAP) score as a measurement of steatosis, ranging from 100 to 400 decibels per meter (dB/m) [3]. Defined cutoffs are used to translate the LSM and CAP scores into estimated degree of fibrosis and percentage of steatosis, respectively [3, 8]. Data on these parameters were originally collected on hepatitis C (HCV) patients, and studies have since shown that VCTE may be used to rule out advanced fibrosis in other liver etiologies [9, 10].
Given these advances in noninvasive methods of detecting steatosis and fibrosis, as well as advances in medical care such as the new effective hepatitis C treatments, indications for liver biopsy have vastly diminished in the past decade. Although noninvasive testing is established in the clinical realm, pathologists may not be aware of the scenarios in which clinicians employ liver biopsy to confirm VCTE findings, and this may lead to uninformative pathology diagnoses. We performed this study to better understand the indications for clinicians to perform a liver biopsy in conjunction with VCTE and to identify discrepancies in steatosis and fibrosis between VCTE and biopsy from a pathology perspective. We also analyzed how often these discrepancies occur and what factors may contribute to discordant findings.
Materials and methods
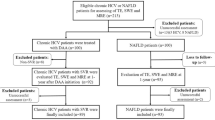
Electronic medical records at a tertiary academic medical center were searched to identify patients who underwent both liver biopsy and VCTE within 1 month of each other, between July 2013 to May 2019 (n = 82). Collected data included clinical histories, indications for VCTE and biopsy, VCTE parameters (probe size, IQR, fibrosis assessment, and CAP steatosis score), liver-related laboratory values (AST, ALT, alkaline phosphatase, total bilirubin, platelet count, albumin level), and demographics. The reported VCTE interpretations were used for data collection with the following cutoffs for fibrosis assessment established at our institution: <8 (F0-2), ≥9 (F3), and ≥12 (F4). Mild steatosis was defined by a CAP between 251 and 299, while moderate to severe steatosis was defined by CAP ≥ 300.
Histologic re-review of the biopsies was performed by a pathologist to confirm diagnosis, degree of fibrosis, and steatosis, as well as to record biopsy length and number of portal tracts. The NASH CRN histological scoring system was used to measure the degree of fibrosis and steatosis [11]. Major discrepancy in fibrosis was defined as F3 or F4 on VCTE, when biopsy showed stage 0–1. Discordance in steatosis was defined as moderate to severe steatosis on VCTE when no or mild steatosis (<33%) was present on the biopsy, or no steatosis on VCTE when severe steatosis (>66%) was seen on biopsy.
ANOVA and chi-square statistical tests were performed using GraphPad Prism, version 8, and statistical significance was defined as p < 0.05.
Results
Eighty-two adult patients (mean age 49 years, range 21–75, 55% female) who had undergone both VCTE and liver biopsy within 1 month of each other were identified (Table 1). The average BMI at the time of VCTE procedure was 30.6 kg/m2 (range 20.16–46.46 kg/m2), and 36.6% of patients had a history of impaired fasting glucose or diabetes. The average FIB-4 scores on the day of the biopsy and day of the VCTE were 2.77 and 2.51, respectively.
The average length of time between VCTE and biopsy was 16.5 days (0–30). Multiple indications for biopsy were often present, the most common being abnormal liver function tests (LFTs) (n = 38), follow up for suspected and/or previously biopsy-proven NAFLD/NASH (n = 31), confirmation of imaging findings that suggested fibrosis (including ultrasound and transient elastography) (n = 28), and suspected drug-induced liver injury (n = 13). Of the patients who were followed for NAFLD/NASH, liver biopsy showed NASH (n = 24), steatosis (n = 3), hepatitis (n = 1), cirrhosis without features of NASH (n = 1), and non-diagnostic (n = 2). Twenty-three (60.5%) of the patients with abnormal LFTs were in those without a previous history of liver disease.
Liver biopsy findings
The average biopsy length was 2.3 cm (range 1–5.5 cm) with an average of 16.7 portal tracts (range 5–34). The distribution of biopsy fibrosis score is as follows: 28.0% with a score of 0 (n = 23), 30.5% score of 1 (n = 25), 7.3% score of 2 (n = 6), 15.9% score of 3 (n = 13), and 18.3% score of 4 (n = 15). The distribution of biopsy steatosis grade was 47.6% (n = 39) for no to minimal steatosis (<5%), 26.8% (n = 22) grade 1 (5–33%), 9.8% (n = 8) grade 2 (33–66%), and 15.9% (n = 13) for grade 3 (>66%).
NASH was seen on biopsy in 43.9% of cases (n = 36) (Table 2). Twenty-two cases showed evidence of hepatitis, including hepatitis C (n = 6), hepatitis B (n = 3), autoimmune (n = 3), drug-induced liver injury (n = 6), and nonspecific liver injury (n = 4). Features of congestion or venous outflow obstruction were identified in 4.9% (n = 4). Cholestatic changes were identified in 11.0% (n = 9), including two cases of primary biliary cholangitis.
VCTE associated factors
To perform VCTE, the M probe size was used more frequently than the XL probe (n = 57 versus n = 25, respectively). The average BMI of a patient for whom an M probe was used was 27.5 kg/m2 (range 16.91–47.54 kg/m2). The average BMI for a patient for whom an XL probe was used was 37.7 kg/m2 (range 28.36–48.0 kg/m2). The interquartile range averaged at 17.7% (range 6–29%). In all of the studies, the patients had a fasting period of at least 3 hours. Four patients had detectable HCV serologies at the time of the VCTE, and one of these had a major fibrosis discrepancy.
The average fibrosis assessment was 15.1 kPa, and the average CAP steatosis score was 272.9 dB/m. The distribution of fibrosis assessment from F0 to F4 is as follows: 28.0% (n = 23) stage F0-F2 (mild hepatic fibrosis), 20.7% (n = 17) stage F3 (probable moderate/severe fibrosis), and 51.2% (n = 42) stage F4 (probable cirrhosis). The distribution of the CAP score steatosis is as follows: no to minimal steatosis (n = 39), probable mild steatosis <33% (n = 10), and probable moderate to severe hepatic steatosis >33% (n = 33).
Factors associated with discrepancy between VCTE and biopsy
Fibrosis by VCTE and biopsy were discrepant in 35.4% (n = 29) (Fig. 1). The VCTE showed advanced fibrosis (F3) in 17 cases and cirrhosis (F4) in 41 cases. However, the liver biopsy demonstrated fibrosis stage 0 or 1 in 50% (n = 29) of these cases. Discordance in fibrosis reading was most associated with BMI > 28 kg/m2 (n = 20/29). Those with both BMI > 28 kg/m2 and NASH also showed frequent discordant findings (n = 15/29) (p-value = 0.035). Categorizing by disease, the majority of discrepancies in fibrosis assessments were in patients with NASH (55.2%, n = 15), followed by those with hepatitis 13.8% (n = 4), cholestasis 17.2% (n = 5), and those with vascular outflow obstruction 10.3% (n = 3). Three cases in the discrepant group had multiple liver diseases, including 1 NASH/hepatitis and 2 cholestasis/hepatitis. Of the 33 patients with elevated ALT >100 IU/L on the day of VCTE, 6 had a major fibrosis discrepancy.
Major discrepancies in fibrosis and/or steatosis by VCTE and liver biopsy are common. In our study, higher BMIs and NASH on biopsy are the most commonly seen factors in both major discrepant fibrosis and steatosis cases. Liver features of cholestasis, congestion, and acute hepatitis are also associated with increased liver stiffness measurements by VCTE.
Steatosis measurements by VCTE and biopsy were discrepant in 21% (n = 18). Thirteen showed mild steatosis (10–33%) on biopsy but “moderate to severe steatosis” on VCTE. Four biopsies showed moderate (33–66%, n = 1) to severe steatosis (>66%, n = 3) and were read as having “no to minimal steatosis” by VCTE. Finally, 1 biopsy showed no steatosis but was reported to have “moderate to severe steatosis” on VCTE. Steatosis discordance was seen most in association with BMI > 28 (94.4%, n = 17) and NASH/NAFLD (88.9%, n = 16). All three discrepant biopsies with severe steatosis (>66%) had features of NASH. 5.6% (n = 1) of discrepant steatosis cases had features of congestion and 5.6% (n = 1) showed hepatitis. No cases of cholestasis had a discrepancy in steatosis.
Discussion
VCTE is a noninvasive tool that can be easily performed in the outpatient setting to evaluate for liver fibrosis and steatosis, and it has been suggested as a viable alternative to liver biopsy [3, 12,13,14]. It is effective for identifying cirrhosis with a negative predictive value of 94–100%, whereas there is higher variability in assessing the severity of fibrosis [3, 15,16,17,18]. There are many factors that contribute to this high variability. VCTE measurements are affected by patient characteristics and procedure performance [3, 19]. Importantly, disruption of the liver matrix, such as significant liver steatosis, inflammation, necrosis, cholestasis, and congestion by vascular outflow obstruction, can especially influence VCTE findings (Fig. 2) [8, 20,21,22,23]. We evaluated liver biopsies from 82 patients who underwent VCTE and liver biopsy within one month of each other to elucidate the clinical scenarios that may require both VCTE and liver biopsy.
Individuals with higher BMIs have an increased distance between the skin and liver capsule, which affects fibrosis assessment because it impacts the VCTE probe’s ability to propagate the shear wave for the evaluation of stiffness. Liver features that disrupt the liver matrix including steatohepatitis, cholestasis, congestion, acute hepatitis, and steatosis have also been described to affect accurate fibrosis assessment by VCTE (hematoxylin-eosin, original magnifications ×200 and ×100).
In our cohort, almost half showed a VCTE and biopsy discrepancy in fibrosis and/or steatosis. All biopsies showing cirrhosis were detected by VCTE; however, VCTE overestimated advanced fibrosis in 35% of our study population. Biopsy was performed specifically to confirm VCTE fibrosis assessment in 24 patients, and 58% of these had a major fibrosis discrepancy. Major fibrosis discrepancies were associated with increased BMI, and patients with both BMI > 28 kg/m2 and NASH on biopsy were more likely to have discordant fibrosis findings. Major steatosis discrepancies occurred in 16% of our cohort and were also associated with increased BMI and NASH on biopsy. Although liver inflammation, cholestasis, and congestion have been found to increase LSM, and therefore increase estimation of fibrosis by VCTE, these factors did not increase the likelihood of a major fibrosis discrepancy in our study (i.e., at least advanced fibrosis on VCTE and no fibrosis on biopsy).
It is useful to know that many factors can influence the results of VCTE and know when a liver biopsy may be ordered to validate VCTE findings. The quality of VCTE is influenced by the total number of measurements, success rate, and variability assessments such as interquartile range to median ratio (IQR/M) [10, 24]. Current recommendations include >10 valid measurements, success rate >60–70%, and an IQR/M < 30% [24, 25]. Other factors that may impact quality include 3 hour fasting prior to the procedure, patient position, probe placement on the body, operator experience, and probe size (M versus XL) [19, 26]. When optimal VCTE parameters are not met or VCTE findings are unexpected, liver biopsy is indicated for diagnosis or confirmation of VCTE findings. All subjects in our cohort met the current recommendations for VCTE, so inadequate VCTE quality was not an indication for liver biopsy in our study.
Obesity is the most well-documented limitation of VCTE, and overestimation of liver fibrosis is common [19]. This is due to the increased distance between the skin and liver capsule and poor propagation of the shear wave through the increased adipose tissue (Fig. 2) [3, 19]. The XL probe was designed to mitigate the higher skin to liver capsule distance by using a lower frequency and larger vibration amplitude to facilitate greater depth of measurement [19, 27, 28]. Studies have shown that using the appropriate probe size based on BMI is unlikely to affect the VCTE reliability, and it has been recommended to switch to the XL probe if there is a high failure rate with the M probe or to start with the XL probe for patients with BMI > 32 to >35 kg/m2 [3, 26,27,28,29]. In our study, the average BMI was 27.5 kg/m2 and 37.7 kg/m2 for the M probe and XL probe, respectively. Even with the XL probe, 38% of patients with BMI > 28 kg/m2 had a major fibrosis discrepancy, and 33% of patients had a steatosis discrepancy. Measurements with the XL probe had increased total discrepancies (fibrosis and/or steatosis), suggesting that the XL probe may not be fully effective in compensating for higher BMI.
Non-alcoholic steatohepatitis is associated with increased BMI, and therefore, discrepancies could be expected in patients with NASH. In our study, major fibrosis discrepancies were seen in 67.6% of liver biopsies with NASH. The NASH activity is based on the presence of steatosis, inflammation, and balloon cells [20, 23]. Significant inflammation and steatosis have individually been identified as contributors that increase LSM, which may explain why the features of steatohepatitis can affect VCTE performance. Coexisting increased BMI (>28 kg/m2) was seen in 88.2% of patients with either steatohepatitis or steatosis only on biopsy, raising the possibility that the trend towards increased discrepancies is related to the BMI. The combination of higher BMI and steatohepatitis was significantly associated with major discrepancies for fibrosis as well as steatosis, suggesting that patients with established NASH are more likely to have a fibrosis discrepancy if they have concurrent increased BMI. In other words, although NASH fibrosis is the key aspect that would benefit from accurate assessment by VCTE, the rate of fibrosis discrepancy between VCTE and biopsy is high in the common scenario of obesity and NASH. Other comorbidities related to increased BMI, such as metabolic syndrome or diabetes mellitus type II, were not significantly associated with higher rates of major discrepancies.
Pathologists should know that liver inflammation has been found to increase LSM, especially when accompanied by necrosis and/or edema. Therefore, VCTE is reportedly less accurate in cases of severe acute hepatitis [19, 21, 30]. This is supported by studies that compared patients with pre- and post-treatment acute hepatitis and found that LSM drastically decreased after appropriate therapy [21, 30,31,32]. In addition, sustained virologic response in hepatitis C patients was also associated with significant decreases in LSM [33, 34]. Clinical markers of inflammation, notably increased ALT, have also been found to correlate with increased LSM [8, 31, 32, 35, 36]. As such, pathologists may receive biopsies to evaluate when a patient has elevated transaminases, such as an ALT > 100 IU/L. In our cohort, 26.8% of patients had active hepatitis of viral, autoimmune, or drug-induced etiology, and about 18.2% of these cases were discrepant. In addition, 40.2% of patients had elevated ALT (>100 IU/L) at the time of VCTE, 33% of which had a major fibrosis discrepancy. Thus, pathologists should be aware that hepatitis and treatment response can result in a discrepancy between VCTE and biopsy, and that clinicians may be asking for biopsy evaluations because of this context.
Congestion or obstructed bile flow can also result in VCTE fibrosis overestimations by causing increased fluid in the liver, and thereby increasing LSMs [19, 20, 37, 38]. Millonig et al. created animal models to mimic congestive heart failure as well as extrahepatic cholestasis and found significant increases in LSM in both models [20, 37]. This corroborated their study of human subjects, in which patients with decompensated congestive heart failure and patients with obstructive jaundice due to neoplastic invasion of the biliary tree had decreases in LSM after diuretics and biliary drainage, respectively [20, 37]. Intrahepatic cholestasis has also been suggested to increase LSM, as bilirubin trends correlated with LSM in patients with acute toxic liver damage, primary biliary cholangitis, or primary sclerosing cholangitis [20, 30]. Therefore, pathologists should be aware that VCTE is not as reliable in patients with a history of hepatic congestion or cholestatic liver diseases. Our study included five patients with liver biopsies that showed features of congestion or venous outflow obstruction, three of which had a major fibrosis discrepancy. We also identified 12 patients with features of cholestasis on biopsy, 5 of which had a major fibrosis discrepancy. When a liver biopsy shows congestion or cholestatic features, a fibrosis discrepancy with VCTE is not unexpected.
Liver steatosis can also be measured by VCTE; however, there are limitations. VCTE is not as precise for steatosis and cannot reliably differentiate moderate steatosis (33–66%) from severe steatosis (>66%); hence, most institutions do not distinguish moderate from severe steatosis [3, 39]. VCTE CAP measurements >250 dB/m typically define at least moderate steatosis (>33%) [3]. Interestingly, exceptionally high CAP values (>323 dB/m) have been unexpectedly associated with increased steatosis discrepancies and paradoxically no longer correlate as well with the degree of steatosis [40]. Factors impacting fibrosis measurements have also been found to affect steatosis [3, 39]. Consistent with these studies, we found that steatosis discrepancies were significantly associated with CAP measurement, BMI, and NASH [39, 40]. In our cohort, VCTE either overestimated or underestimated steatosis in 21% of patients. While VCTE successfully detected all liver biopsies with severe fibrosis and cirrhosis, VCTE missed 4 of 10 cases of moderate to severe steatosis. It is thus useful to understand that VCTE is not as sensitive nor specific for detecting steatosis. Its primary strength in the outpatient setting is its ability to detect advanced fibrosis and cirrhosis. Biopsy evaluation for steatosis and steatohepatitis can help add important information for the patient’s management.
The most common indication for ordering the biopsy in our study population was to confirm fibrosis findings on imaging (VCTE and ultrasound, n = 24 and 4, respectively). Furthermore, there were 31 patients who had at least one other VCTE that was not associated with a liver biopsy, presumably for surveillance of their liver disease. We also saw both tests ordered to evaluate elevated LFTs in patients without a history of liver disease (n = 23). From a pathology perspective, while the liver biopsy is traditionally performed to establish an initial diagnosis, we will now see an increase in biopsies to confirm the findings of noninvasive screening tools.
In summary, pathologists should be aware that they may receive biopsies to confirm the fibrosis and steatosis results of noninvasive screening methods. Moreover, discrepancies regarding both are not uncommon. Therefore, liver biopsy still plays an important role in the accurate diagnosis and management of patients. Increased BMI has been associated with discrepancies due to the increased skin-to-liver capsule distance seen in obesity. It is also helpful to be aware that factors that disrupt the liver matrix, notably significant steatosis, inflammation, necrosis, and edema, impact VCTE fibrosis scores as well. Clinicians utilize VCTE as a screening method for its high sensitivity and noninvasiveness, which may decrease the number of liver biopsies in patients who need lifelong surveillance. A discrepant result between VCTE and biopsy may raise undue concern to patients and clinicians who are less familiar with VCTE. In these situations, pathologists can play an impactful advisory role by providing reassurance regarding the factors that contribute to discrepancies. Because VCTE can inaccurately overestimate fibrosis, liver biopsy is still the gold standard for confirmation of liver fibrosis.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Oseini A, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (Nash). Liver Int. 2017;37:97–103.
Pandyarajan V, Gish RG, Alkhouri N, Noureddin M. Screening for nonalcoholic fatty liver disease in the primary care clinic. Gastroenterol Hepatol. 2019;15:357–65.
Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–81.e1264.
Machado MV, Diehl AM. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology. 2016;150:1769–77.
Perumpail B, Khan M, Yoo E, Cholankeril, G Kim, D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263–76.
Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95.
Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–13.
Del Poggio, P, Colombo, S. Is transient elastography a useful tool for screening liver disease? World J Gastroenterol. 2009;15:1409–14.
Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156–63.e152.
Mendes LC, Ferreira PA, Miotto N, Zanaga L, Goncales ESL, Pedro MN, et al. Elastogram quality assessment score in vibration-controlled transient elastography: diagnostic performance compared to digital morphometric analysis of liver biopsy in chronic hepatitis C. J Viral Hepat. 2018;25:335–43.
Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: Definitions, risk factors, and workup. Clin Liver Dis. 2012;1:99–103.
Younossi Z, Loomba R, Anstee Q, Rinella M, Bugianesi E, Marchesini G, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68.
Hashemi SA, Alavian SM, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Casp J Intern Med. 2016;7:242–52.
Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2:100067.
Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease—the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharm Ther. 2014;39:254–69.
Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–501.
Friedrich–Rust M, Ong M-F, Martens S, Sarrazin C, Bojunga J, Zeuzem S. et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–74.e968.
Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013–21.
Wilder J, Patel K. The clinical utility of FibroScan® as a noninvasive diagnostic test for liver disease. Med Devices. 2014;7:107–14.
Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Buchler MW, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718–23.
Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380–4.
Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato M, Ronchi G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–73.
Shen F, Mi Y, Xu L, Liu Y, Wang X, Pan Q, et al. Moderate to severe hepatic steatosis leads to overestimation of liver stiffness measurement in chronic hepatitis B patients without significant fibrosis. Alimentary pharmacol. Therap. 2019;50.
Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182–91.
Jang HW, Kim SU, Park JY, Ahn SH, Han KH, Chon CY, et al. How many valid measurements are necessary to assess liver fibrosis using FibroScan® in patients with chronic viral hepatitis? An analysis of subjects with at least 10 valid measurements. Yonsei Med J. 2012;53:337–45.
Berger A, Shili S, Zuberbuhler F, Hiriart JB, Lannes A, Chermak F, et al. Liver stiffness measurement with FibroScan: use the right probe in the right conditions! Clin Transl Gastroenterol. 2019;10:e00023.
Durango E, Dietrich C, Seitz HK, Kunz CU, Pomier-Layrargues GT, Duarte-Rojo A, et al. Direct comparison of the FibroScan XL and M probes for assessment of liver fibrosis in obese and nonobese patients. Hepat Med. 2013;5:43–52.
Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–71.
Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30.
Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592–5.
Tapper E, Cohen E, Patel K, Bacon B, Gordon S, Lawitz E, et al. Levels of alanine aminotransferase confound use of transient elastography to diagnose fibrosis in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2012;10.
Hartl J, Denzer U, Ehlken H, Zenouzi R, Peiseler M, Sebode M, et al. Transient elastography in autoimmune hepatitis: timing determines the impact of inflammation and fibrosis. J Hepatol. 2016;65.
D’Ambrosio R, Aghemo A, Fraquelli M, Rumi M, Donato M, Paradis V, et al. The diagnostic accuracy of fibroscan for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol. 2013;59.
Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440–51.e446.
Alhankawi D, HunJung K, Sharma S, Weinberger J, Park J. Transient elastography (Fibroscan) compared to FIB-4, APRI,…. Am J Gastroenterol. 2018;113:S556.
Coco B, Oliveri F, Maina A, Ciccorossi P, Sacco R, Colombatto P, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepatitis. 2007;14:360–9.
Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206–10.
Hopper I, Kemp W, Porapakkham P, Sata Y, Condon E, Skiba M, et al. Impact of heart failure and changes to volume status on liver stiffness: non-invasive assessment using transient elastography. Eur J Heart Failure. 2012;14.
Jun B, Park W, Park E, Jang J, Jeong S, Lee S, et al. A prospective comparative assessment of the accuracy of the FibroScan in evaluating liver steatosis. PLoS ONE. 2017;20.
Jung K, Kim B, Kim S, Chon Y, Chun K, Kim S, et al. Factors affecting the accuracy of controlled attenuation parameter (CAP) in assessing hepatic steatosis in patients with chronic liver disease. PLoS ONE. 2014;9.
Acknowledgements
We would like to thank Robin G. Kunkel, MS. for her artwork and figures.
Author information
Authors and Affiliations
Contributions
JMF and MW performed study concepts and designs; JMF, JC, MFC, JA, and MW performed development of methodology and writing, review, and revision of the paper; JMF, JC, and MW provided acquisition, analysis and interpretation of data, and statistical analysis; All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval/consent to participate
At the time of surgery, patients at the University of Michigan consent to tissue blocks and slides being archived for use in future research. This study on archival resected tissue blocks and slides was approved by the University of Michigan Institutional Review Board (IRB) (study ID# HUM00143268). Additional consent was waived by the IRB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, J.M., Cheng, J., Chang, M.F. et al. Transient elastography versus liver biopsy: discordance in evaluations for fibrosis and steatosis from a pathology standpoint. Mod Pathol 34, 1955–1962 (2021). https://doi.org/10.1038/s41379-021-00851-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00851-5