Abstract
Breast implant-associated anaplastic large cell lymphoma (ALCL) is a distinctive type of T-cell lymphoma that arises around textured-surface breast implants. In a subset of patients, this disease can involve surrounding tissues, spread to regional lymph nodes, and rarely metastasize to distant sites. The aim of this study was to assess sequential pathologic specimens from patients with breast implant-associated ALCL to better understand the natural history of early-stage disease. To achieve this goal, we searched our files for patients who had breast implant-associated ALCL and who had undergone earlier surgical intervention with assessment of biopsy or cytologic specimens. We then focused on the patient subset in whom a definitive diagnosis was not established, and patients did not receive current standard-of-care therapy at that time. We identified a study group of ten patients with breast implant-associated ALCL in whom pathologic specimens were collected 0.5 to 4 years before a definitive diagnosis was established. A comparison of these serial biopsy specimens showed persistent disease without change in pathologic stage in three patients, progression in five patients, and persistence versus progression in two patients. Eventually, six patients underwent implant removal with complete capsulectomy and four underwent partial capsulectomy. Seven patients also received chemotherapy because of invasive disease, three of whom also received radiation therapy, two brentuximab vedotin after chemotherapy failure, and one allogeneic stem cell transplant. Eight patients achieved complete remission and two had partial remission after definitive therapy. At time of last follow-up, six patients were alive without disease, one had evidence of disease, one died of disease, and two patients died of unrelated cancers. In summary, this analysis of sequential specimens from patients with breast implant-associated ALCL suggests these neoplasms persist or progress over time if not treated with standard-of-care therapy.
Similar content being viewed by others
Introduction
Breast implant-associated anaplastic large cell lymphoma (ALCL) was first described in 1997 [1], although it is likely that this disease occurred earlier [2], and this neoplasm is recognized as an entity in the World Health Organization classification [3, 4]. Breast implant-associated ALCL is uncommon, and current estimates suggest that one woman will develop this disease for every 355 to 30,000 patients who receive textured-surface breast implants [5,6,7]. Most of the earliest described patients with breast implant-associated ALCL presented with effusion surrounding breast implants, causing swelling, capsular sclerosis, and pain or discomfort [8, 9]. Subsequently, patients with breast implant-associated ALCL were described who presented with a mass lesion as a result of the neoplasm infiltrating peri-implant capsules or extra-capsular invasion into surrounding tissues [10]. Uncommonly, regional lymph node involvement has been observed [11], with axillary lymph nodes most often involved by breast implant-associated ALCL. In rare situations, patients have developed widespread systemic disease including bone marrow involvement [12, 13].
The heterogeneity of clinical presentations and oncologic outcomes and the clinically indolent nature of breast implant-associated ALCL has resulted in debate regarding the clinical course of patients with early-stage breast implant-associated ALCL, and consequently the appropriate treatment for these patients. For example, Kadin et al. [14] speculated that disease manifesting as a simple effusion may regress spontaneously if left untreated, similar to cutaneous lymphoproliferative disorders that can undergo spontaneous regression. However, in our experience breast implant-associated ALCL is a slowly progressive disease. In a study of 87 patients with breast implant-associated ALCL by Clemens et al. [15] we observed a high rate of recurrence and disease progression in patients with breast implant-associated ALCL who presented with effusion-only disease and had received only fine needle aspiration.
In this study, our goal is to address the natural history of breast implant-associated ALCL in its earliest stages. We searched our files for patients with breast implant-associated ALCL, who in their past underwent pathologic evaluation of biopsy or cytologic fluid specimens, at which time a diagnosis of breast implant-associated ALCL was not established, and curative therapy that consists of implant removal and complete capsulectomy with negative margins was not administered. These sequential specimens were only available for patients who did not undergo timely treatment, either due to a “missed” diagnosis or to the patient deciding to postpone appropriate therapy for personal reasons.
Materials and methods
In this collaborative study from several institutions, we searched for patients diagnosed with breast implant-associated ALCL between January 1, 1998 through December 31, 2019 and focused on patients who had undergone earlier procedures such as incomplete capsulectomy, aspiration of peri-implant fluid, or biopsy. This initial selection led to the identification of 46 patients. For this group, 36 patients did not undergo pathologic examination and were excluded from this study. The remaining ten patients underwent pathologic evaluation, but received only a descriptive diagnosis, not diagnostic of lymphoma, and therefore patients did not receive appropriate therapy as defined for current standard of care. These ten cases formed the study cohort, and six of these patients have been published in the literature [8, 10, 15,16,17].
All routinely stained and immunohistochemistry slides (when performed) from these earlier pathologic or cytologic specimens were obtained and reviewed. Pathologic stage was determined using criteria described previously [15, 18]. For this study, we assessed all specimens using immunohistochemical analysis and an antibody specific for CD30. Tris-EDTA buffer was used as antigen retrieval, with a CD30 Ber-H2 clone, prediluted ready to use, from Cell Marque Millipore, Rocklin, California, USA [18].
Clinical data were collected by review of the medical records and included age, gender, reason for implant, type and texture of implant, time from implantation to lymphoma, signs and symptoms at initial presentation as well as at the time lymphoma was diagnosed, surgical procedure at initial presentation, use of subsequent surgical or adjuvant therapy, and clinical follow-up. Time from implantation to lymphoma diagnosis was defined as the interval between the first breast implant surgery and the time of definitive lymphoma diagnosis, regardless of whether patients had undergone implant revisions. The Institutional Review Board at MD Anderson Cancer Center and at collaborative institutions approved this study.
Results
The median age of patients in the cohort at time of definitive diagnosis of breast implant-associated ALCL was 55.5 years. These patients developed breast implant-associated ALCL 6 to 35 years after initial implant placement (Table 1). Implants were filled with silicone in seven and saline in three patients. Information regarding the implant surface was provided in seven patients, all textured. A remote history of effusion surrounding implants was documented in nine patients, and one case was identified incidentally in a patient undergoing surgery for breast carcinoma (Table 2).
All ten patients had invasive procedures performed a median of 1 year (range, 0.6–4 years) before a subsequent procedure that yielded a definitive diagnosis of lymphoma. The earlier procedures performed included capsular sampling/incision/partial excision, implant replacement, or fluid aspiration. The diagnoses rendered for these earlier specimens were descriptive and all specimens were diagnosed as negative or inconclusive for lymphoma.
However, our review of the earliest pathologic specimens from these patients showed breast implant-associated ALCL. The lymphoma was likely missed because the neoplastic infiltrates were subtle, and the tumor burden was difficult to assess because of necrosis and fibrosis. In these earlier specimens the diagnosis of breast implant-associated ALCL was confirmed retrospectively by immunohistochemistry using an anti-CD30 antibody, positive in all cases (Figs. 1–4). The pathologic stage of disease in the initial specimens was T1 (n = 2), T2 (n = 4), T3 (n = 1), T4 (n = 1), and indeterminate (n = 2). Pathologic stage was indeterminate in two patients; only effusion fluid was obtained originally in these patients whose imaging studies revealed thickening of the capsule, but no evidence of a mass.
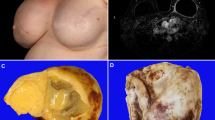
Section of capsule shows pathologic stage T1, similar to initial biopsy specimen (A: H&E, ×200; inset ×400). The neoplastic cells appear confined to the luminal side of the capsule, circumscribed by underlying fibrous tissue (B. CD30 immunohistochemistry with hematoxylin counterstain, ×200; inset ×400).
The low magnification (A, H&E, ×40) shows thickening of the capsule, and two large vaguely nodular lesions, one of which is shown at intermediate magnification, and reveals a cellular lesion with central necrosis (B, H&E, ×100). High magnification shows a mixed infiltrate of large cells and inflammatory cells (C, H&E, ×400). Detail of the infiltrate shows scattered large cells with vesicular nuclei and clear cytoplasm admixed with small lymphocytes and eosinophils (D, H&E, ×1000). Immunohistochemistry with anti-CD30 highlights the large cells that appear as a large aggregate (E, immunohistochemistry with hematoxylin counterstain, ×100). CD30 reacts with neoplastic cells in a membrane and Golgi pattern (F, immunohistochemistry with hematoxylin counterstain, ×400).
At the time a definitive diagnosis of breast implant-associated ALCL was established, the morphologic features of the neoplasm were more apparent. The pathologic stage of these neoplasms was T2 (n = 1), T3 (n = 2), T4 (n = 6), and T4 with lymph node involvement (n = 1). Compared with the earlier specimens, three patients had the same pathologic stage, and five had a higher pathologic stage. The two patients with initial indeterminate pathologic stage had pathologic stage T4 at the time of definitive diagnosis, suggesting that these patients had persistent disease (Table 2).
Upon establishing the diagnosis of breast implant-associated ALCL, six patients underwent complete capsulectomy and four partial capsulectomy. Seven patients received chemotherapy that consisted of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or CHOP-like chemotherapy regimens; three of these patients also received radiation therapy, two brentuximab vedotin, and one allogeneic stem cell transplant. One patient with pathologic stage 4 disease underwent partial capsulectomy, had persistent disease, and died at last follow-up. Four women who developed clinically significant mass lesions, including one who also had lymph node involvement, received chemotherapy or radiation therapy; two of these patients received brentuximab vedotin achieving only partial responses. The median follow-up time from definitive treatment of breast implant-associated ALCL to last follow-up was 4 years (range, 2–15 years). At last follow-up, six patients were alive without evidence of disease, one was alive with disease, one died of disease, and two died of unrelated cancers (one diffuse large B-cell lymphoma, and one small cell carcinoma of the lung).
Discussion
We identified ten patients who underwent surgical procedures or aspiration of peri-implant fluid a median of 1 year before a definitive diagnosis of breast implant-associated ALCL was established. Since the initial specimens were not diagnosed as being involved by breast implant-associated ALCL, and consequently these patients did not receive standard-of-care therapy, the disease likely followed its natural history until the diagnosis was established definitively. Specifically, this case series allows us to assess the natural history of breast implant-associated ALCL in patients with early clinical stage disease, which in every patient persisted or progressed. This study also confirms a previous observation that many patients (>80% in our series) [15] with breast implant-associated ALCL undergo surgical procedures or therapeutic drainage of the peri-implant fluid without pathologic examination of these tissue or fluid specimens. This finding supports the notion that patients with breast implants who develop symptoms are often not worked up for the possibility of breast implant-associated ALCL, leading to a delayed diagnosis and potentially disease progression, as seen in at least five patients in this cohort. Furthermore, we show that the diagnosis of breast implant-associated ALCL can be missed in at least a subset of patients who undergo invasive procedures, likely due to lack of awareness of the disease, the extensive tumor necrosis, or if not suspected, the large cells may be interpreted as degenerative reactive changes.
Others have suggested that breast implant-associated ALCL can undergo spontaneous regression after peri-implant fluid aspiration [19]. This suggestion is based on the apparent decrease of neoplastic cells in patients who presented with effusion, initially underwent fluid aspiration with pathologic detection of disease, and subsequent complete capsulectomy showed decreased or almost no lymphoma cells. Others have considered this observation to be flawed because patients received therapy within a few months of fluid aspiration [20]. Although it is difficult to prove that something does not happen, and therefore the findings in this study do not entirely exclude the possibility of spontaneous regression, we suggest that the clinically indolent nature of the disease, and possibly under sampling for pathologic evaluation [21], might be misconstrued as spontaneous regression. We suggest that the advanced stage of disease that occurs in rare patients with breast implant-associated ALCL substantiates the World Health Organization classification in which breast implant-associated ALCL is considered to be a bona fide lymphoma [22]. We also believe that acceptance of the idea of spontaneous resolution of breast implant-associated ALCL puts forward a concept that, at a minimum, is misleading regarding the potential severity of this disease, and could delay diagnosis or result in under-treatment, resulting in greater morbidity or possibly mortality for these patients. We acknowledge, however, the limited number of patients we report, and the need to compare the results we report with the experience of other investigators. Along these same lines of thought, Brody et al. [23] have suggested that even when invasion of the capsule is present, delaying surgical resection will not affect prognosis because breast implant-associated ALCL behaves as an “exceedingly indolent entity”. Brody et al. [23], however, did not provide adequate data for the basis of this assertion. On the contrary, Clemens et al. [15] demonstrated that depth of invasion has prognostic significance, and Ferrufino-Schmidt et al. [11] showed that invasion by lymphoma correlates with regional lymph node involvement. We therefore agree that breast implant-associated ALCL is clinically indolent in most patients, but we have concerns about the suggestion that delaying proper therapy will not affect prognosis. In this study, two patients experienced disease progression within 1 year. In another patient, after an initial specimen was misdiagnosed as benign, treatment was delayed for ~3 years, at which time she presented with advanced stage disease and lymph node involvement. Therefore, we adhere to current recommendations and guidelines [24] that clinicians need to send fluid specimens or capsule biopsy specimens obtained from patients who have symptoms attributable to their breast implants for pathologic examination, and pathologists need to examine these specimens thoroughly to identify the possible presence of breast implant-associated ALCL, as the data we present support the value of early treatment.
During the clinical follow-up interval after definitive treatment, no patients developed recurrent disease, consistent with the recommendation that complete capsulectomy is essential for the cure of breast implant-associated ALCL [15]. Regarding the proposed role of textured implants in the pathogenesis of breast implant-associated ALCL, the data in this study show that all patients in whom the surface characteristics were known had textured implants. These data are in keeping with the decision by the United States Food and Drug Administration to recall the Biocell textured breast implants in July of 2019, which was involved in 91% of worldwide cases in which the device history was known [25].
In summary, we have assessed sequential biopsy specimens in ten patients with breast implant-associated ALCL, all of whom had a biopsy or fluid specimen examined up to 4 years before a definitive diagnosis was established. Retrospective review of the earlier specimens showed lymphoma that was subtle and had been “missed.” These patients did not receive adequate initial management at that time, thereby allowing us to observe the natural history of breast implant-associated ALCL. The data presented suggest that early stage of breast implant-associated ALCL persists or progresses over time. Enhanced awareness is essential among physicians from several specialties, who must recognize that breast implant-associated ALCL appears to be a lymphoma at its onset and is likely to persist or progress over time. The results of this study also support those of earlier studies indicating that definitive treatment of early-stage disease is beneficial and can minimize future morbidity from breast implant-associated ALCL.
Data availability
Data are confidential and not available to review by external sources.
References
Keech JA Jr., Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–55.
Lyapichev KA, Medeiros LJ, Clemens MW, Ferrufino-Schmidt MC, Marques-Piubelli ML, Chai SM, et al. Reconsideration of the first recognition of breast implant-associated anaplastic large cell lymphoma: a critical review of the literature. Ann Diagn Pathol. 2020;45:151474.
Miranda RN, Feldman AL, Soares FA. Breast implant-associated anaplastic large cell lymphoma. In: Allison KH et al., editors. World Health Organization breast tumours. Lyon: IARC; 2019. p. 245–48.
Feldman AL, Harris NL, Stein H, Campo E, Kinney MC, Jaffe ES, et al. Breast implant-associated anaplastic large cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: IARC; 2017. p. 421–2.
Doren EL, Miranda RN, Selber JC, Garvey PB, Liu J, Medeiros LJ, et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017;139:1042–50.
Loch-Wilkinson A, Beath KJ, Knight RJW, Wessels WLF, Magnusson M, Papadopoulos T, et al. Breast implant-associated anaplastic large cell lymphoma in Australia: a longitudinal study of implant and other related risk factors. Plast Reconstr Surg. 2019;140:645–54.
Cordeiro PG, Ghione P, Ni A, Hu Q, Ganesan N, Galasso N, et al. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstr Aesthet Surg. 2020;73:841–6.
Aladily TN, Medeiros LJ, Amin MB, Haideri N, Ye D, Azevedo SJ, et al. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol. 2012;36:1000–8.
Roden AC, Macon WR, Keeney GL, Myers JL, Feldman AL, Dogan A. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-cell lymphoproliferative disorder. Mod Pathol. 2008;21:455–63.
Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, De Jong D, Fayad LE, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32:114–20.
Ferrufino-Schmidt MC, Medeiros LJ, Liu H, Clemens MW, Hunt KK, Laurent C, et al. Clinicopathologic features and prognostic impact of lymph node involvement in patients with breast implant-associated anaplastic large cell lymphoma. Am J Surg Pathol. 2018;42:293–305.
Loghavi S, Medeiros LJ, Javadi S, Lin P, Khoury JD, Nastoupil L, et al. Breast implant-associated anaplastic large cell lymphoma with bone marrow involvement. Aesthet Surg J. 2018;38:NP92-6.
Zimmerman A, Locke FL, Emole J, Rosa M, Horna P, Hoover S, et al. Recurrent systemic anaplastic lymphoma kinase-negative anaplastic large cell lymphoma presenting as a breast implant-associated lesion. Cancer Control. 2015;22:369–73.
Kadin ME, Adams WP Jr, Inghirami G, Di Napoli A. Does breast implant-associated ALCL begin as a lymphoproliferative disorder? Plast Reconstr Surg. 2020;145:30e–8.
Clemens MW, Medeiros LJ, Butler CE, Hunt KK, Fanale MA, Horwitz S, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34:160–8.
Loddenkemper C, Anagnostopoulos I, Hummerl M, Johrens-Leder K, Foss HD, Jundt F, et al. Differential Emu enhancer activity and expression of BOB.1/OBF.1, Oct2, PU.1, and immunoglobulin in reactive B-cell populations, B-cell non-Hodgkin lymphomas, and Hodgkin lymphomas. J Pathol. 2004;202:60–9.
Farkash EA, Ferry JA, Harris NL, Hochberg EP, Takvorian RW, Zuckerman DS, et al. Rare lymphoid malignancies of the breast: a report of two cases illustrating potential diagnostic pitfalls. J Hematop. 2009;2:237–44.
Quesada AE, Medeiros LJ, Clemens MW, Ferrufino-Schmidt MC, Pina-Oviedo S, Miranda RN. Breast implant-associated anaplastic large cell lymphoma: a review. Mod Pathol. 2019;32:166–88.
Fleming D, Stone J, Tansley P. Spontaneous regression and resolution of breast implant-associated anaplastic large cell lymphoma: implications for research, diagnosis and clinical management. Aesthetic Plast Surg. 2018;42:672–8.
Magnusson MR, Deva AK. Letter to Editor: Fleming D, Stone J, Tansley P. Spontaneous Regression and Resolution of Breast Implant-Associated Anaplastic Large Cell Lymphoma: Implications for Research, Diagnosis and Clinical Management. Aesthetic Plast Surg. 2018;42:1164–6.
Lyapichev KA, Pina-Oviedo S, Medeiros LJ, Evans MG, Liu H, Miranda AR, et al. A proposal for pathologic processing of breast implant capsules in patients with suspected breast implant anaplastic large cell lymphoma. Mod Pathol. 2020;33:367–79.
Collins MS, Miranda RN, Medeiros LJ, Silva de Meneses MP, Iyer SP, Butler CE, et al. Characteristics and treatment of advanced breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2019;143:41s–50.
Brody GS, Deapen D, Taylor CR, Pinter-Brown L, House-Lightner SR, Andersen JS, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;135:695–705.
Jaffe ES, Ashar BS, Clemens MW, Feldman AL, Gaulard P, Miranda RN, et al. Best practices guideline for the pathologic diagnosis of breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2020;38:1102–11.
Anonymous. FDA takes action to protect patients from risk of certain textured breast implants. 2019. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-patients-risk-certain-textured-breast-implants-requests-allergan. Accessed 29 Mar 2020.
Funding
Department of Hematopathology at The University of Texas MD Anderson Cancer Center.
Author information
Authors and Affiliations
Contributions
All authors were involved in the design, writing, and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was been approved by the Institutional Review Board. Patients consented to participate in research study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Evans, M.G., Medeiros, L.J., Marques-Piubelli, M.L. et al. Breast implant-associated anaplastic large cell lymphoma: clinical follow-up and analysis of sequential pathologic specimens of untreated patients shows persistent or progressive disease. Mod Pathol 34, 2148–2153 (2021). https://doi.org/10.1038/s41379-021-00842-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00842-6