Abstract
The severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) pandemic has had devastating effects on global health and worldwide economy. Despite an initial reluctance to perform autopsies due to concerns for aerosolization of viral particles, a large number of autopsy studies published since May 2020 have shed light on the pathophysiology of Coronavirus disease 2019 (COVID-19). This review summarizes the histopathologic findings and clinicopathologic correlations from autopsies and biopsies performed in patients with COVID-19. PubMed and Medline (EBSCO and Ovid) were queried from June 4, 2020 to September 30, 2020 and histopathologic data from autopsy and biopsy studies were collected based on 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A total of 58 studies reporting 662 patients were included. Demographic data, comorbidities at presentation, histopathologic findings, and virus detection strategies by organ system were collected. Diffuse alveolar damage, thromboembolism, and nonspecific shock injury in multiple organs were the main findings in this review. The pathologic findings emerging from autopsy and biopsy studies reviewed herein suggest that in addition to a direct viral effect in some organs, a unifying pathogenic mechanism for COVID-19 is ARDS with its known and characteristic inflammatory response, cytokine release, fever, inflammation, and generalized endothelial disturbance. This study supports the notion that autopsy studies are of utmost importance to our understanding of disease features and treatment effect to increase our knowledge of COVID-19 pathophysiology and contribute to more effective treatment strategies.
Similar content being viewed by others
Introduction
The severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) infection has affected most countries worldwide, with 34,026,003 confirmed cases and 1,015,107 deaths estimated by the World Health Organization as of October 1st, 2020 [1]. SARS-CoV-2 is a new member in the betacoronavirus genus of the family of Coronaviridae [2,3,4,5,6]. Coronavirus disease-2019 (COVID-19) caused by human-to-human transmission occurs by virus contact with oral, nasal, or ocular mucosa [2], commonly by inhalation of small droplets exhaled by an infected person [4, 7]. SARS-CoV-2 infects the host by binding to the angiotensin-converting enzyme 2 (ACE2) receptor with its structural spike glycoprotein (S protein) [2, 6, 8, 9]. The ACE2 receptor is localized on several human tissues, including pulmonary, renal, cardiovascular, and gastrointestinal epithelia, and is expressed by arterial and venous endothelial cells and arterial smooth muscle cells [10], making them potential targets for SARS-CoV-2 infection [2].
SARS-CoV-2 has an overall lower case fatality rate (0.0–29%), although variable by country, than that of Severe Acute Respiratory Syndrome coronavirus (SARS, 9.5%) and Middle East Respiratory Syndrome coronavirus (MERS, 34.4%), but more efficient and aggressive viral transmission [2, 4, 11,12,13,14,15,16]. Although the clinical course of COVID-19 ranges from an asymptomatic condition to severe multi-organ failure leading to death, respiratory tract flu-like manifestations, such as fever and dry cough, are the most commonly reported symptoms [3,4,5,6, 11, 12, 17,18,19]. Elderly patients, males, and subjects with comorbidities, such as obesity, diabetes, hypertension, and cardiovascular disease, appear most affected and have a worse prognosis [3, 4, 11, 12, 17,18,19].
Few autopsies were initially performed due to concern surrounding aerosolization and infectivity of the virus. Moreover, most initial autopsies in COVID-19 patients were limited, often restricted to sampling of lungs [20, 21]. More recently, in compliance with biosafety recommendations of several international regulatory agencies, including the World Health Organization [22], the Centers for Disease Control and Prevention (CDC) [23], and the European Centre for Disease Prevention and Control [24], rapidly expanding autopsy literature has become available. This review will summarize the current histopathologic findings in COVID-19 derived from autopsy and biopsy studies.
Methods
Eligibility criteria, search strategy, study selection criteria, manuscript quality evaluation, and data collection are reported in the Supplemental Material.
Results
A total of 58 articles describing 662 patients, met inclusion criteria. At the time of this manuscript preparation, we analyze 508 decedents from 43 studies, including 121 full autopsies with or without brain (14 studies), 76 limited autopsies (6 studies), 17 full and 4 limited autopsies (1 study), 7 standard autopsies and 7 in situ dissections (1 study), 63 full, 3 limited and 2 biopsies (1 study), 13 minimally invasive autopsies (MIA) (2 studies), 1 in situ dissection (1 study), 18 postmortem biopsies (5 studies), and 176 autopsies with modality unspecified (NOS) (12 studies); and 151 living patients (14 studies), including 148 biopsies (12 studies) and 3 surgical specimens (2 studies). Autopsies from 2 decedents and a surgical specimen from 1 survivor were included together in 1 study.
The quality analysis revealed that 18 (31%) articles included in this review were of high quality, 25 (43%) were of moderate quality, and 15 (26%) were of low quality (Supplementary Table 1).
The demographic findings and the pathologic characteristics are summarized in Tables 1 and 2, respectively.
Lung
COVID-19 lung injury has been described in 263 cases from 28 studies, including 138 full autopsies, 73 limited autopsies, 14 combined in situ and standard autopsies, 3 surgical resections, 5 postmortem biopsies, 10 ultrasound-guided MIAs, 1 in situ dissection, 1 transthoracic needle biopsy, and 19 autopsies NOS [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
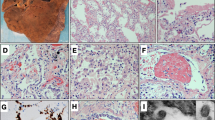
An early study in 2 patients undergoing lobectomy for lung adenocarcinoma and retrospectively found to have SARS-CoV-2 infection at surgery showed exudative and proliferative acute lung injury with edema, inflammatory infiltrate, type 2 pneumocyte hyperplasia, and interstitial and/or focal intra-alveolar organization, but no obvious hyaline membrane formation. These findings significantly contribute to our understanding of the early findings in COVID-19 [34]. Frank diffuse alveolar damage (DAD) was reported in 230 cases [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] (Fig. 1A, B), including acute [26,27,28,29,30,31, 35, 36, 38, 41, 43, 45, 46, 51], acute and proliferative/organizing [29, 32, 37, 40, 41, 43, 44], proliferative [28, 29, 31, 34, 36, 41, 43, 47, 49, 51], proliferative/organizing and fibrotic [32] and fibrotic DAD [28]. Detailed findings included squamous metaplasia [27, 29, 30, 37, 44, 50, 51], pneumocyte reactive hyperplasia [27, 29,30,31, 34, 36, 37, 39, 41, 45, 47,48,49,50,51,52], multinucleated giant cells [29, 31, 34,35,36,37, 45, 51], interstitial lymphocytic infiltrate [26,27,28,29,30,31, 35,36,37, 40, 41, 43,44,45,46,47,48,49, 52] (Fig. 1C), interstitial/alveolar edema [26, 28, 29, 31, 34, 35, 37, 39, 41, 45, 46, 48, 51, 52], alveolar/interstitial thickening [34, 36, 37, 41, 47,48,49, 51], arteriolar microthrombi [25,26,27,28,29,30,31, 37, 41, 44, 46, 47, 50, 51], capillary megakaryocytes [27, 28, 37, 44], alveolar hemorrhage [27, 29, 31, 36, 37, 44, 48], necrotizing and non-necrotizing vasculitis [29, 33, 45, 51], and organizing pneumonia with intra-alveolar organizing exudates [29, 48, 50, 51] (Fig. 1D). Other findings included acute pneumonia with intra-alveolar neutrophilic inflammation, described as suggestive or indicative of aspiration or secondary infection [26, 28, 29, 37, 40, 41, 43]. Granulocytic infiltration of the alveoli and bronchi, resembling bacterial focal bronchopneumonia [30], areas of neutrophilic inflammation ranging from focal bronchial or bronchiolar inflammation to acute bronchopneumonia [31], granulocyte-dominated focal confluent bronchopneumonia [38], suppurative pneumonia [44], acute and chronic alveolar space inflammation [50], bilateral lobar pneumonia [52], bronchial/bronchiolar inflammation [26, 29,30,31, 40, 41, 46], and tracheobronchitis [29, 46, 50] were also described. These changes were isolated or in association with DAD.
A Normal lung with open alveoli and delicate alveolar septa containing thin capillaries lined by an attenuated alveolar epithelium (hematoxylin-eosin; original magnification ×200). B Acute diffuse alveolar damage (DAD) with hyaline membranes lining alveolar spaces, pneumocyte hyperplasia, desquamation of alveolar epithelial cells into the alveolar spaces, inflammatory infiltrates, and capillary congestion (hematoxylin-eosin; original magnification ×200). C Perivascular inflammation (hematoxylin-eosin; original magnification ×400). D Organizing pneumonia with granulation tissue plugs within the lumen of respiratory bronchioles (hematoxylin-eosin; original magnification ×200).
Viral cytopathic effect (CPE) has been described in type 2 pneumocytes [28], in pneumocytes of unspecified type [35, 45, 50, 52], and alveolar epithelial cells [44]. Atypical enlarged multinucleated and syncytial pneumocytes are commonly seen in COVID-19 lungs and have also been previously described in SARS, MERS, and other viral infections affecting lungs. These have been attributed to viral CPE in type 2 pneumocytes/alveolar epithelial cells by some authors [53,54,55]. Viral inclusions were also reported [36, 48].
SARS-CoV-2 has been detected in 48 of 53 lungs by RT-PCR [26, 29,30,31, 36, 51, 52], 29 of 43 by immunohistochemistry (IHC) [31, 40, 41, 43, 49, 50], 13 of 23 by in situ hybridization (ISH) [50], 3 of 8 by next-generation sequencing (NGS) [41] and 6 of 6 by culture [50]. Viral protein was found by IHC in alveolar pneumocytes (variably, type 1, 2 or both) [31, 41, 43, 49, 50], alveolar macrophages [40, 41, 43], hyaline membranes in acute DAD [41, 43], tracheobronchial respiratory epithelium [31, 41, 43, 50], and minimally in endothelial cells [41, 49]. Similarly, by ISH, viral RNA was identified in tracheal epithelium, hyaline membranes, and type 2 pneumocytes [50]. Timing from onset of symptoms affects detection. IHC identified SARS-CoV-2 nucleocapsid protein in acute phase (≤7 days) but not organizing DAD (>14 days from the onset of respiratory failure). Remdesivir’s role in one of these two patients is unclear from this small study [43]. Similarly, viral detection by IHC, ISH, and NGS was more likely within the initial 10 days to 2 weeks from onset [50] and in acute as opposed to proliferative DAD. Viral protein and SARS‐CoV‐2 viral RNA were detected by IHC and NGS, respectively, in pneumocytes and early but not late hyaline membranes, possibly reflecting viral clearance [41]. Variable detection may also be associated with the type of SARS-CoV-2 antibody (nucleoprotein or spike protein) [41] and with viremia, as patients without viremia showed no or a low virus load [30]. Using RT-PCR, higher RNA copy numbers were detected in lung tissue than in brain, heart, testicle, and kidney tissue [29].
A few studies have demonstrated SARS-CoV-2 virions by transmission electron microscopy (TEM) within endothelial cells [56], type 2 pneumocytes [37, 56, 57], extracellularly associated with upper airway ciliated cells, and within alveolar macrophage phagosomes [57]. However, putative SARS– CoV-2 virions have been purportedly reported by TEM in other studies (Supplementary Table 2).
Heart
The heart has been evaluated in 19 postmortem studies, including 52 full and 13 limited autopsies, 1 limited in situ autopsy, 3 postmortem biopsies, 14 in situ and standard autopsies, 10 ultrasound-guided minimally invasive biopsies, and 90 autopsies NOS for a total of 184 patients [26,27,28,29,30,31,32,33, 35, 36, 39, 40, 42, 44, 45, 52, 58,59,60], and 3 endomyocardial biopsy studies in 7 surviving patients [61,62,63]. Pre-existing injury associated with hypertension and coronary artery disease, such as myocardial hypertrophy or interstitial fibrosis, are the most common findings in many studies [27, 29,30,31,32, 36, 45]. Amyloidosis was identified in 10–28% of patients [29, 31, 62]. Acute findings attributed to SARS-CoV-19 infection included acute myocarditis [30,31,32, 39, 40, 44, 59, 62], individual myocyte necrosis [29, 31, 58, 59], borderline myocarditis [62, 63], inflammatory cardiomyopathy [62], epicardial lymphocytic infiltrate [27, 32, 40, 59], lymphocytic endothelialitis [33], focal interstitial lymphocytic infiltrate [35, 45, 59], interstitial macrophages [59], and recent myocardial infarction [29, 40, 59]. Fibrin microthrombi with or without megakaryocytes within the cardiac microvasculature [40, 59] and intramyocardial and epicardial venous thrombosis were identified [40]. Viral genome was detected by RT-PCR in 39 [30, 31, 36, 60, 63] of 66 patients [30, 31, 36, 51, 60, 62,63,64]. IHC using a monoclonal antibody to the spike protein was negative in one tested patient [31]. ISH detected viral RNA within interstitial cells, but the number of cases tested was not specified [60].
Kidney
A detailed pathologic examination of kidney injury in COVID-19 is highlighted in 22 studies including 194 patients. Kidney histopathology was available in 189 patients (1 patient was reported in 2 studies), including 9 (full and limited) autopsy studies [26, 27, 29, 30, 33, 39, 40, 65, 66], 1 study with combined standard autopsy and in situ dissection [31], 3 studies with minimally invasive/in situ limited dissection [44, 45, 47] and 9 renal biopsy studies [67,68,69,70,71,72,73,74,75].
Excluding glomerular lesions attributable to pre-existing injury, such as diabetic nephropathy and arteriosclerosis/arterionephrosclerosis, the most common finding in COVID-19 is acute tubular injury with tubular dilation, epithelial cell sloughing with luminal debris, and cytoplasmic vacuolization with occasional necrosis [29, 31, 39, 40, 44, 45, 65,66,67,68,69,70,71,72,73,74,75]. Frank cortical necrosis [47, 73], pigmented tubular casts/rhabdomyolysis [44, 66, 69, 73], acute pyelonephritis [66], thrombotic microangiopathy with endothelial injury, and glomerular or arteriolar fibrin thrombi were additional findings [27, 29, 40, 44, 65, 66, 73] (Fig. 2A), but parenchymal infarct was a rare event [65, 69].
A Thrombotic microangiopathy with glomerular capillary loop and arteriole fibrin thrombi (hematoxylin-eosin; original magnification ×400). B Glomerulus with segmental capillary collapse and overlying podocyte hyperplasia (Jones methenamine silver; original magnification ×400). C Small bowel thrombus and mucosal ischemic changes (hematoxylin-eosin; original magnification ×100). D Spleen hilar thrombus (hematoxylin-eosin; original magnification ×200). E Detail of Fig. D with transmural vascular inflammation (hematoxylin-eosin; original magnification ×400). F Splenic thrombosis and infarct (hematoxylin-eosin; original magnification ×100).
Immunofluorescence (IF) showed negative or nonspecific IgM and C3 in 36 of 45 cases. TEM was performed in 54 cases. Fibrinogen positivity within fibrin thrombi (1 case) [65], IgA nephropathy (1 case) [65], membranous glomerulopathy (2 cases), lupus nephritis (1 case), anti-glomerular basement membrane glomerulonephritis (1 case) [69], crescentic glomerulonephritis [73], segmental granular capillary IgG without C3 (but with scattered humps observed by EM, described in the EM section below), and mesangial and capillary IgA (with corresponding mesangial and subendothelial deposits seen by EM) [66] have been described.
Deposits were described in 3 cases, 1 with IgA nephropathy [65], and 2 with paramesangial, subendothelial, and subepithelial “hump-like”, and scant isolated mesangial deposits [66]. Minimal change disease [69] and collapsing glomerulopathy have been described in 19 patients (1 patient reported in 2 different series) [69, 70], of whom 18 were African American and 1 was African [65, 67,68,69,70,71, 73,74,75] (Fig. 2B). In 1 case, tubular microcysts accompanied the glomerular lesion, reminiscent of HIV-associated nephropathy. In these reports, 15 of 19 patients tested for APOL1 gene abnormalities had G1/G1, and 6 had G1/G2 high-risk alleles.
SARS-CoV-2 IHC/IF showed viral protein in tubules in 3 of 32 cases [66, 69, 73]. ISH detected viral RNA with a manual but not an automated test in 2 of 33 cases [65, 69, 70, 75]. RNA was positive in 8 of 15 patients by RT-PCR [30, 31], negative in 3 by Nanostring analysis [75] and in 1 by RT-PCR [72], and yielded a low copy number by RT-PCR in 16 cases [29].
Gastrointestinal tract
The gastrointestinal tract (GI) is a potential target of SARS-CoV-2 due to ACE2 receptor expression by the GI mucosa [10, 76]. GI symptoms, including diarrhea, nausea, vomiting, anorexia, and abdominal pain [11, 77] have been described in 3–61.1% of COVID-19 patients [11, 77, 78] either at illness onset or during hospitalization [77]. Subjects with GI symptoms have a longer interval from illness onset to hospital admission, and symptoms become more pronounced with disease progression [78]. About 3% of patients present only with GI symptoms and without respiratory complaints [78,79,80].
The lower GI (LGI) tract is more likely to be involved than upper GI (UGI); thus, we report the autopsy finding separately. Of note, detailed postmortem histological examination of the GI tract often lacks in the literature, owing to more focused descriptions of lung histopathology [20] and challenges of autolytic changes. Viral RNA detected in GI tissue by RT-PCR generally correlates with disease severity and is more reliable than RNA detected in the stool [77].
Upper gastrointestinal tract (UGI)
UGI postmortem examination has been reported in 22 cases from 5 studies comprising 12 full autopsies [26, 31, 81, 82], 3 MIAs [83] and 7 in situ dissections [31]. Although most cases had neither significant gross nor histological pathological changes in esophagus, stomach or duodenum [82, 83], gaseous stomach, punctate gastric and duodenal hemorrhages, and multifocal gastric hemorrhage have been described [26, 31, 81], although with unclear etiologic relationship to SARS-CoV-2, and corresponding to the endoscopic findings of 4–6 mm bleeding and round ulcers, sometimes fused and covered with what has been termed “white moss” in COVID-19 patients [77].
Lower gastrointestinal tract (LGI)
LGI postmortem findings have been reported in 8 studies including 98 full [26, 30, 33, 38, 39, 81, 82] and 3 MIAs [83]. The most common finding is a lack of intestinal abnormality [26, 39, 81, 82]. Chronic inflammation [26, 83], shock [38], and ischemic changes in the small bowel have been described [84] (Fig. 2C). Endothelialitis and apoptotic bodies have been reported in 2 autopsies and in the resected small intestine of a survivor. In 2 of 3 cases, mesenteric ischemia led to small bowel resection [33]. Similar findings of enterocolitis associated with ischemic colitis have been reported in 3 of 12 postmortem examinations [30].
Intestinal ischemic damage has been described during endoscopic and surgical procedures and confirmed with histologic examination [84,85,86]. Other histological features included apoptotic bodies and endothelial hobnail and bizarre nuclear shapes resulting from direct viral CPE, identified by IHC for the nucleocapsid protein of SARS-CoV-2 [84].
Liver
Hepatic injury with elevated liver enzymes and total bilirubin and decreased albumin has been described in up to 44% of COVID19 patients [11, 87,88,89]. Abnormal liver function is associated with disease severity [88, 89], worse pulmonary involvement [88], and prolonged hospital stay [87].
The liver has been analyzed in 164 patients from 19 studies, including 123 full autopsies, 13 MIAs, 7 in situ dissections, 8 post-mortem biopsies and 13 autopsies NOS [26, 27, 29,30,31,32,33, 35, 36, 38, 39, 44, 81,82,83, 88, 90,91,92]. The most frequent postmortem histological features are centrilobular congestion [30, 31, 38, 39, 44, 82, 90], most likely attributable to shock [44], and mild to moderate steatosis, predominantly macrovesicular and centrilobular [26, 27, 29,30,31, 35, 39, 44, 88, 90, 92], and probably associated with preexistent obesity and diabetes mellitus [44]. Hepatocyte necrosis has been described in 23 cases with individual cell, multifocal, or massive distribution [29, 31, 33, 36, 39, 44, 83, 92]. Clustered or scattered apoptotic hepatocytes and binuclear or occasionally multinuclear syncytial hepatocytes with apparent nucleolus and condensed chromatin have been observed in 2 postmortem biopsies [88]. Mild to moderate lymphocytic infiltrate is present in the periportal zone [27, 31, 32, 35, 36, 39, 44, 83, 88, 91]. Cholestasis with canalicular bile plugs and nuclear pleomorphism of cholangiocytes have been described in 10 and 9 cases, respectively [39, 83].
Although RT-PCR for detection of SARS-CoV-2 has not frequently been performed in postmortem samples, 10 of 31 cases resulted positive with high titers in 6 [30, 36], and low in 4 [31, 81, 82]. No SARS-CoV-2 RNA was found in the bile of 4 patients [39].
Pancreas
High plasma levels of amylase or lipase have been reported in 9 of 52 (17%) patients [93, 94], 6 of whom (67%) also exhibited moderate hyperglycemia [93]. Many COVID-19 patients have diabetes [95], but whether this is associated with death remains controversial [96]. Patients with alterations of pancreatic enzymes have a higher incidence of GI symptoms, more severe illness on admission, lower levels of CD3+ and CD4 + T cells, higher liver enzymes, and higher erythrocyte sedimentation rate compared to subjects without altered values [93].
The pancreas has not been commonly investigated in COVID-19 postmortem examinations. Current available data refer to 6 studies with 44 patients, including 34 full autopsies, 3 MIAs, and 7 in situ dissections [26, 30, 31, 39, 82, 83] with most reports showing no significant abnormalities [26, 30, 31, 82]. Degeneration of a few pancreatic islet cells without abnormalities in the exocrine pancreas [83] and asymptomatic focal pancreatitis in 5 of 11 patients (45%) [39] have been reported. However, severe pancreatitis has been rarely reported in clinical studies [97]. An additional autopsy study published during the review process of this manuscript reports pancreatitis in 2 of 8 (25%) patients, 1 frankly necrotic-hemorrhagic and another with microscopic acute inflammation but without macroscopic abnormality [98]. These findings may represent a direct viral effect [93], consistent with ACE2 receptor expression in the exocrine and endocrine pancreas [94], an indirect effect of respiratory failure, or a harmful immune response induced by SARS-CoV-2 infection [93].
Spleen and lymph nodes
Lymphocytopenia has been reported in up to 83.2% of COVID-19 patients, especially elderly, subjects with comorbidities, and severe disease [4, 11, 93, 99, 100]. Total T cell counts, CD8 + T cells, or CD4 + T cells lower than 800, 300, or 400/μL, respectively, have been associated with poor survival [99].
Spleen and lymph nodes have been investigated in several postmortem examinations [26, 27, 29,30,31,32, 38, 39, 44, 81,82,83, 92, 101], providing evidence of a variety of tissue injury patterns. However, SARS-CoV-2 RNA and viral antigens’ detection are controversial and rarely demonstrated in lymphoid organs [27, 31, 81, 82].
The spleen has been examined in 13 studies from 161 patients, including 123 full and limited autopsies, 8 MIAs, 7 in situ dissections, 10 post-mortem biopsies, and 13 autopsies NOS [26, 27, 30,31,32, 38, 39, 44, 81,82,83, 101, 102]. Postmortem findings include lymphocytes depletion [27, 39, 44, 83, 101], white pulp atrophy [27, 31, 39, 101], parenchymal necrosis [5, 83], and vascular involvement including congestion, hemorrhage, infarction [27, 30, 38, 39, 44, 82, 101], vasculitis and arterial thrombosis [44, 101] (Fig. 2D–F). Acute splenitis [29, 30, 44], neutrophil infiltration [101], splenomegaly lacking lymphoid follicles and showing necrotizing granulomas [82], and hemophagocytosis have also been described [81, 101].
Lymph nodes have been investigated in 45 patients from 8 studies, including 33 full autopsies, 5 MIAs, and 7 in situ dissections [26, 29, 31, 39, 44, 81, 82, 92]. Postmortem examination revealed diffuse congestion with sinus dilatation, mainly in mediastinal, perihilar, and paratracheal lymph nodes [29, 39]. Histologic changes include decreased total lymphocytes with absence of germinal centers [39, 44], increased reactive plasmablasts in the interfollicular zone [29], and hemophagocytosis [31, 44, 81]. Rarely necrotizing granulomas have been reported in peribronchial lymph nodes [82].
Skin
Cutaneous manifestations have been described in up to 20.4% of COVID-19 patients, mainly in trunk and limbs [4, 103,104,105,106,107,108,109,110,111,112,113,114,115,116]. Clinical presentation most commonly includes erythematous rash [4, 104,105,106, 108, 111, 116] widespread urticaria [104, 112,113,114], varicella-like papulovesicular exanthem [104, 108, 113, 116], acrocyanosis, chilblains and other acral lesions [103, 109, 110, 114, 115, 117], livedoid eruptions [106, 107, 109] (Fig. 3), and purpura [105,106,107, 109].
Histopathologic examinations of survivors’ skin biopsies reveal a superficial and deep perivascular infiltrate of lymphocytes or neutrophils with some eosinophils or occasional plasma cells [105, 108,109,110,111, 117]. Epidermal alterations include apoptotic, necrotic, and dyskeratotic keratinocytes, occasionally resembling ballooning herpes-like cells [106, 108, 110, 113, 117], in some cases associated with vacuolar alteration, [110, 111, 113, 117] and focal acantholytic suprabasal clefts [108]. The most frequent dermal vascular lesions are thrombotic vasculopathy with fibrinoid or inflammatory thrombi and endothelial cell injury [106,107,108,109, 117]. Sweat gland necrosis, more evident in the secretory portion of the eccrine sweat coil and with preserved eccrine ducts, is described [109, 117]. Deposition of terminal complement components (C5b-9 and C4d) by IHC has been demonstrated in samples taken from cutaneous lesions and normal-appearing skin. Moreover, 1 case showed co-localization of C4d with SARS-CoV-2 spike glycoprotein within the cutaneous microvasculature [107]. RT-PCR and SARS-CoV-2 cultures were negative in skin lesion samples of other cases [109, 111].
Skin examination has been reported in 16 autopsies, of which 3 full and 13 MIAs from 4 studies [44, 82, 83, 91]. Many of these reports describe normal cutaneous layers and appendages, and mild perivascular lymphocytic infiltrate and petechiae [83, 91] with negative RT-PCR [82]. Dermatitis and a hypercoagulative status, possibly directly related to viral infection, were reported in 1 study. Superficial dermis perivascular mononuclear infiltrate in 11 of 13 cases [44, 83], dermis and hypodermis small vessels endothelial changes and fibrin microthrombi in 3 cases [83], and purpura in 1 case [44] were also described. These autopsy findings are consistent with the cutaneous manifestations described clinically and histopathologically in survivors.
Central nervous system
Autopsies documenting central or peripheral nervous system involvement in COVID-19 are scarce. Of 148 total autopsies from 12 studies including brain dissection, description of neuropathologic findings are available in 109 patients [27, 29, 31, 32, 39, 43, 44, 118,119,120,121,122]. Of these autopsies, 15 were full, 10 were ultrasound-guided MIAs, and 84 were NOS. In most instances, brain autopsy findings were unremarkable or showed chronic features without acute morphologic alteration [27, 31, 32, 39, 122]. Major histopathologic features were acute hypoxic injury with neuronal loss [29, 43, 119, 121], encephalitis [120], lymphocytic meningitis [120], petechial bleeding [120], perivascular hemorrhage [44, 118, 121], axonal injury/degeneration [120, 121], white matter clusters of macrophages [118, 121], perivascular acute disseminated encephalomyelitis-like appearance [121], focal microscopic areas of necrosis with central loss of white matter and marked axonal injury [121], astrogliosis [122], lymphocytic infiltrates [122], and microscopic infarcts [121, 122]. SARS-CoV-2 nucleocapsid protein was identified in 9 of 26 brains by quantitative RT-PCR [29, 118, 119] but not by IHC [118, 119]. In 4 of 4 patients, copy numbers were generally low compared to other organs, but the olfactory bulb values were higher than those in the brainstem [29]. Interestingly, the cause of death in 3 of 6 patients younger than 65 was massive intracranial hemorrhage or pulmonary embolism. These patients exhibited diffuse petechial hemorrhage in the entire brain, and both groups of patients who died of vascular and cardiorespiratory causes showed lymphocytic pan-encephalitis and meningitis. Changes were seen in hippocampus, neocortex, cerebellum, and brainstem nuclei [120].
Coagulation alterations
Pulmonary embolism (PE) is a cause of death in a subset of COVID-19 patients. PE was described in 27 of 115 patients, of whom 39, including those with PE, had deep venous thrombosis [29, 30, 38, 39, 42].
Pulmonary thrombosis in small and medium-size pulmonary artery branches [39], as well as multi-organ thrombosis [42], was identified in 18 of 18 autopsies, of which 8 had associated pulmonary infarction [39]. Of note, 14 patients had received anticoagulation, suggesting that pulmonary thrombi formed despite treatment [39, 40]. Similarly, no significant association was found between anticoagulation therapy and presence of thrombi, with 46% (22 of 48) of anticoagulated patients having large thrombi and 88% (42 of 48) having arteriolar and capillary microthrombi in another study [50].
Multisystem inflammatory syndrome in children (MIS-C)
Postmortem examination of an 11-year-old child with MIS-C demonstrated heart failure as the primary determinant of fatal outcome with myocarditis, pericarditis, and endocarditis [123]. In this case, SARS-CoV-2 was detected in heart tissue by RT-PCR and, purportedly by TEM. In the fatal case of a 17-year-old, the inflammatory infiltrate present within the heart was eosinophil-rich [90].
Discussion
The pathophysiologic mechanism of COVID-19 is still poorly understood. Autopsies have significantly contributed to our knowledge of the pathologic derangement occurring with COVID-19 and provided evidence for current treatment strategies. It is apparent from the growing number of autopsy studies that SARS-CoV-2 direct effects are primarily limited to the lung, while the ensuing systemic disease occurs through indirect rather than direct effects. This discussion will focus on the main systemic aspects of the disease and hypothesize a unifying pathogenic mechanism.
The spectrum of respiratory involvement in COVID-19 is broad, ranging from asymptomatic infection to flu-like symptoms to variably severe pneumonia, multi-organ failure, and death. In cases with a progressive course, acute respiratory distress syndrome (ARDS) develops in 31–41.8% of COVID-19 patients. Mortality among those who develop ARDS is 52.5–93% [18, 124]. The clinical definition of ARDS is based on Berlin criteria [125, 126], and mild, moderate, and severe ARDS is associated with increased mortality (27%; 32%; and 45%). Most patients meeting clinical criteria for ARDS have histologic features of DAD [127,128,129]. ARDS may be caused by infection/sepsis, shock, trauma, aspiration, transfusion, drug reaction, oxygen toxicity, vaping-associated pulmonary injury, and connective tissue disease, but the pathognomonic histologic lesion is the same—hyaline membranes occurring as a response to alveolar and endothelial injury, causing capillary endothelial damage and leakage of plasma into the alveolar space [130]. This exudate admixed with cellular debris lines the alveolar surface impeding regular gas exchange. DAD progresses from an acute (exudative) phase characterized by hyaline membranes into an organizing/proliferative phase, lasting 1–3 weeks and characterized by interstitial proliferation of fibroblasts and myofibroblasts, type 2 pneumocyte hyperplasia, and squamous metaplasia. If the patient survives the acute and proliferative stage, there may be resolution, stabilization of the process, or progression to the fibrotic (chronic) phase with collagen deposition, architecture remodeling, and honeycomb lung. Residual functional impairment is the consequence of continued interstitial fibrosis and airspace remodeling. In typical DAD caused by various etiologies, microvascular thrombi are part of the spectrum of pathologic lesions, and extensive vascular remodeling may be seen [130, 131]. A contribution of antibody-dependent enhancement (ADE) is being actively investigated in COVID-19. A role for ADE has been invoked in SARS-CoV and MERS infection, among other viral infections. Immune complexes resulting from ADE mechanisms could contribute to DAD [132].
In our study, 152 of 263 (58%) patients had pathologic evidence of interstitial pneumonia (morphologically defined by interstitial lymphocytic infiltrate), and 50 (19%) had acute pneumonia or bronchopneumonia. Aspiration or superimposed bacterial infection were likely the underlying etiology of acute pneumonia, based on the neutrophilic pattern of infiltration. Characteristic morphologic patterns of viral injury in the lung include interstitial inflammation, DAD, and necrotizing bronchitis/bronchiolitis [53]. In our study, 230 of the total 263 patients had DAD, and 152 had interstitial lymphocytic infiltrate/pneumonia, described as sparse in some cases. Previous studies have shown that bilateral pneumonia is both a cause and a mimicker of ARDS. In a pre-COVID-19 ARDS autopsy study, 58% of patients had pneumonia, while 42% had DAD only without pneumonia, and 36% of clinically suspected ARDS were histologically diagnosed as pneumonia, while 20% of cases clinically diagnosed as pneumonia had only DAD on autopsy [133]. Early ARDS studies show that sepsis with multi-organ failure is the cause of death in 84% of patients, while respiratory failure accounts for only 16% of fatalities [134,135,136,137]. A systemic inflammatory response with fever, elevated inflammatory markers (eg, D-dimer, ferritin), and release of the proinflammatory cytokines tumor necrosis factor, interleukin (IL)-1, IL-6, and IL-8 exceeding the body homeostatic mechanisms may determine a DAD injury and a systemic response independently of the presence of pneumonia, similar to that seen in some severe cases of COVID-19.
Thromboembolism is another emerging feature of COVID-19. Ackermann et al. compared lung autopsy findings in COVID-19, influenza A (H1N1), and uninfected controls. Although both cohorts shared similar DAD findings, COVID-19 patients had severe endothelial injury with disrupted endothelial cell membranes and more arterial but less venous vascular thrombosis than influenza patients. COVID-19 and influenza patients had greater expression of lung ACE2 compared to uninfected controls and more ACE2-positive endothelial cells and endothelial injury [25]. Heterogenous disease stages and treatment applied to both cohorts make interpretation of these results complex [138]. The issue of thromboembolism versus thrombosis in COVID-19 has been debated in recent literature [139,140,141]. Lax et al. distinguish thrombosis from thromboembolism based on subsegmental artery involvement, distribution of thrombotic material in multiple vessels, associated endothelialitis, subtotal or total filling of the occluded vessels, and distribution of thrombotic events in areas of hypostasis, as opposed to the randomly distributed pattern of thromboembolism [39, 141]. Micro- and macrothrombosis of pulmonary arteries have been described with endothelialitis [51]. It is apparent that vascular thrombosis is a frequent phenomenon in COVID-19 autopsies. However, a thrombosis secondary to local inflammation frequently accompanies DAD secondary to diverse etiologies [130, 138, 142, 143]. Pulmonary infarcts are also seen with intubation in ARDS [140, 144]. These findings suggest the pulmonary microthrombi may not be specific to COVID-19 but are associated with ARDS. Thus, definitive pathogenetic distinction between coagulopathy, endotheliopathy, and vasculitis, and whether these are a result of direct viral effect or systemic inflammation does not emerge from these autopsy studies. In autopsies from COVID-19 inpatients, COVID-19 untreated decedents in the community, and SARS-CoV-2 negative DAD controls, all but 1 untreated community COVID-19 patient with focal fibrinous pneumonia had DAD. COVID-19 inpatient and community patients had similar lung histopathologic findings at autopsy, suggesting that DAD is a viral and not iatrogenic injury [145]. DAD in COVID-19 patients was histologically indistinguishable from DAD from other causes. Thrombosis was similar in SARS-CoV-2 positive patients and negative controls [145]. COVID-19, 2003 SARS, and H1N1 showed DAD in 88%, 98%, and 90% of patients, respectively, while 57% of COVID-19, 58% of SARS, and 24% of H1N1 influenza patients had pulmonary microthrombi [146], suggesting that these findings are not specific for COVID-19. Persistence of multisystemic thrombosis at autopsy in a subset of COVID-19 patients undergoing prophylactic inpatient anticoagulation suggests that microthrombosis may be secondary not only to a generalized endothelial disturbance but also to an inflammatory response with cytokine release, fever, and inflammation, which is known to be characteristic of ARDS [64, 147, 148]. Additional larger comparative studies are required to further clarify this issue, shed light on the use of prophylactic versus therapeutic anticoagulation and associated risk of complications, and prevent the systemic inflammatory response before the ineluctable chain of events caused by the cytokine storm is initiated.
Nonspecific shock injury in multiple organs was the main finding in this review. This injury is described in the GI system and kidney, occasionally accompanied by vasculitis or thromboembolic events. A prospective study of 701 patients admitted to a COVID-19 tertiary hospital in Wuhan showed that acute kidney injury occurred in 5.1% of patients [149]. This is reflected by the nearly global finding of acute tubular injury in our study. However, in the same study, 43.9% also had proteinuria, and 26.7% had hematuria [149]. African ancestry is a known risk factor for kidney disease, resulting from G1 and G2 risk alleles in the APOL1 gene [150]. Collapsing glomerulopathy is strongly associated with APOL1 risk alleles [151]. Although the pathogenic role of APOL1 in kidney diseases is still unclear, collapsing glomerulopathy in the setting of APOL1 risk alleles homozygosity has been associated with HIV nephropathy, lupus nephritis, membranous glomerulopathy, interferon and pamidronate treatment [152,153,154,155,156]. Glomerular and inflammatory conditions, such as the COVID-19 cytokine storm, could result in a “second hit” injury superimposed on genetic susceptibility due to APOL1 risk variants [157, 158].
Hepatic injury in COVID-19 may be multifactorial, related to direct viral CPE, hypoxic damage, cytokine storm, sepsis, or drug hepatotoxicity [89, 159,160,161]. Moreover, patients may have underlying chronic liver disease, such as cirrhosis, hepatitis, or cancer, which may increase the risk of SARS-CoV-2 infection with immunosuppression [89, 159] and contribute to more severe liver damage, particularly with concurrent hypoxia [161]. Therefore, it is important to distinguish acute pathologic changes attributed to direct SARS-CoV-2 infection from chronic underlying diseases potentially predisposing to a fatal COVID-19 course and nonspecific secondary effects related to hypoxia, hypotension, or sepsis [39].
Acute myocardial injury, defined by elevated cardiac troponin levels, has been clinically described in 5–38% of COVID-19 patients [162, 163]. Patients with comorbidities or cardiovascular disease are more likely to present with cardiac symptoms. Myocardial injury is associated with a greater need for mechanical ventilation and higher in-hospital mortality [164]. Although a clinically significant component of COVID-19, myocardial injury appears to be limited in autopsy studies. A myocarditis meeting histopathological Dallas criteria was only identified in 27 of 191 patients (14%) in our study. Similarly, limited myocardial injury not meeting Dallas criteria, e.g., individual myocyte necrosis, focal myocardial lymphocytic infiltrate, or “borderline myocarditis” (the latter two forms representing the same entity but often separately described in a few studies) were identified in 16 (8%), 8 (4%), and 5 (3%) patients, respectively. SARS-Cov-2 genome in heart tissue was detected in 60% of our reviewed cases, but IHC for the spike protein was negative in 1 tested patient. Therefore, systemic inflammation secondary to cytokine release, endothelial inflammation, and associated microvascular thrombosis leading to multi-organ failure may be the main pathogenetic mechanisms associated with COVID-19 myocardial injury [164]. The long-term effects of COVID-19 myocardial injury are yet to be established. However, in pre-COVID studies, death for biopsy-proven viral myocarditis ranged from 19% at 5 years to 39% at 10 years, respectively [165, 166].
Ischemic stroke, encephalopathy, meningoencephalitis, acute necrotizing encephalitis, and Guillain-Barrè syndrome have been clinically documented in COVID-19 [167,168,169,170,171,172]. Although still limited, autopsy data seem to support a contribution of metabolic derangement in critically ill patients associated with hypoxemia and a systemic proinflammatory status, typical of COVID-19. SARS-Cov-2 genome has been demonstrated in 34% of brains, supporting a direct viral effect.
Two patterns of cutaneous COVID-19 manifestations have been identified. Exanthems, such as rash, urticarial eruptions, and chickenpox-like vesicles tend to occur early in the disease course and sometimes precede other systemic manifestations [114, 173]. Vascular lesions, such as chilblains, livedoid eruptions, and purpura, usually appear several days after the onset of general symptoms or even in their absence [114, 173]. The first group of lesions could represent an early response to the initial viral replication or the cytokine storm. In contrast, the second could result from a delayed cell-mediated immunologic response to the virus or an unbalanced coagulation state towards a prothrombotic microenvironment with microthrombi formation in the dermal vessels. The early cutaneous manifestations could aid in the prompt identification of asymptomatic patients, improve epidemiological tracking, and promote timely therapeutic management. The skin vascular lesions might represent a marker of systemic vessel injury. Therefore, these patients’ prognosis could improve after administration of anticoagulant and anti-inflammatory therapy.
In children, COVID-19 symptomatology is typically milder [174]. However, in April 2020, several children with no prior medical history presenting with fever, cardiovascular shock, and hyperinflammation syndrome were reported [175,176,177,178,179,180]. In May 2020, the CDC issued an advisory to report cases meeting criteria for multisystem inflammatory syndrome in children (MIS-C) [181], defined by fever and severe illness requiring hospitalization with multisystem (≥2) organ involvement, laboratory evidence of inflammation, and recent infection or exposure to SARS-CoV-2 within four weeks prior to symptoms onset in individuals aged <21 years. MIS-C is rare, with an estimated incidence of 2 per 100,000 [182]. As of September 17, 2020, a total of 935 confirmed cases with 19 deaths were reported in the US.
There are clinical and pathophysiologic similarities in MIS-C and Kawasaki disease (KD), a febrile illness affecting young children characterized by vasculitis that can result in coronary artery aneurysms. In two series of MIS-C, 73–78% of children were previously healthy, with the most common comorbidities being obesity and asthma [183, 184]. Similar to KD, MIS-C is a post-infectious phenomenon related to IgG-ADE rather than the result of acute viral infection [178, 185,186,187]. A predominantly gastrointestinal presentation (92%) [187] is frequent, while respiratory symptoms (70%) are often due to severe shock, and ARDS is not a prominent feature [184]. Acute cardiac decompensation [188] and cardiovascular system involvement were present in 80% of patients with coronary artery aneurysms in 8% [183]. In contrast, MIS-C and KD differ in several aspects, including ethnicity, age of onset, clinical and immunologic manifestations. While ~80% of children with KD are predominantly of Asian descent and younger than five years (peak incidence around 10 months of age), children with MIS-C are predominantly of Hispanic and Afro-Caribbean descent and have a median age of 8.3–11 years [183, 184]. In KD, coronary artery abnormalities are more frequent than in MIS-C, occurring in 15–25% of patients and decreasing to 5% with prompt therapy, while these occur only in 1% of MIS-C cases. Left ventricle dysfunction and shock are also more likely in the latter. Immunologically, KD has an interleukin17A-mediated hyperinflammation, which is absent in MIS-C, and higher levels of biomarkers associated with arteritis and coronary artery disease. Furthermore, MIS-C has lower naïve CD4 + T cell and T follicular helper and increased central and effector memory subpopulations compared to KD [189]. MIS has also been reported in adults recently, although as a rare occurrence [190,191,192].
Putative SARS-Cov-2 virions or viral-like particles suggestive of SARS-Cov-2 have been purportedly identified by TEM in several recent reports. These highly contested reports [56, 102, 193,194,195,196,197,198,199,200] often describe physiological structures and organelles, such as clathrin-coated vesicles, multivesicular bodies, rough endoplasmic reticulum, and unidentified subcellular structures. This issue highlights the challenging task of identifying SARS-Cov-2 virions by TEM due to unfamiliarity with coronavirus morphology and morphogenesis, artifacts of delayed postmortem fixation, lack of detection due to possible viral clearing, and sensitivity of the methodology, as reviewed by Hopfer et al., and Bullock et al. [102, 201].
Limitations
Limitations of this study include its focus on COVID-19 histopathologic injury rather than laboratory, imaging, and macroscopic findings. In addition, disparate reporting methodologies emerged from the analyzed studies, accounting for vastly heterogeneous data in the literature. We chose to include only peer-reviewed articles and exclude pre-prints. Thus, some articles may have been missed if the initial pre-print was subsequently published. Similarly, given the fast pace of recent publications emerging on this topic, articles published during the editing and review stages of this manuscript were not included.
Conclusions
In summary, SARS-CoV-2 viral protein has been identified in situ only in a small proportion of extrapulmonary organs in a subset of patients, supporting a limited extrapulmonary direct effect of the virus. The predominant pathologic findings emerging from this review suggest that a unifying mechanism underlying the systemic clinical manifestation of COVID-19 is the characteristic inflammatory response of ARDS with cytokine release, fever, inflammation, generalized endothelial disturbance, and secondary multisystemic shock-injury. Although very preliminary, an additional contributing factor could be a detrimental immunologic response, such as ADE, which is being actively investigated in COVID-19.
Autopsies have reaffirmed their value to public health,aiding our understanding of novel diseases. Though limitations due to postmortem autolytic changes may hinder some studies, the increasing autopsy literature supports a wealth of information contributing to our understanding of COVID-19.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
World Health Organization. World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. 2020. Accessed 5 July 2020.
Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci. 2020;16:1678–1685.
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20.
Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606.
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20.
Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020. https://doi.org/10.1001/jama.2020.12458.
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–92 e286.
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80 e278.
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of areport of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42.
de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–34.
Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11.
Johns Hopkins Unuversity. Coronavirus Resource Center. Mortality analyses. 2021. https://coronavirus.jhu.edu/data/mortality Accessed 2 Apr 2021.
Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20:e238–e244.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–43.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–9.
Salerno M, Sessa F, Piscopo A, Montana A, Torrisi M, Patane F et al. No autopsies on COVID-19 deaths: a missed opportunity and the lockdown of science. J Clin Med. 2020;9.
Barth RF, Xu X, Buja LM. A call to action: the need for autopsies to determine the full extent of organ involvement associated with COVID-19. Chest. 2020;158:43–44.
World Health Organization. Infection prevention and control for the safe management of a dead body in the context of COVID-19: interim guidance, 24 March 2020. https://apps.who.int/iris/handle/10665/331538 Accessed 5 July 2020.
Centers for Disease Control and Prevention (CDC). Collection and submission of postmortem specimens from deceased persons with known or suspected COVID-19, March 2020 (Interim guidance) 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html Accessed 5 July 2020.
European Centre for Disease Prevention and Control. Considerations related to the safe handling of bodies of deceased persons with suspected or confirmed COVID-19. 2020. https://www.ecdc.europa.eu/en/publications-data/considerations-related-safe-handling-bodies-deceased-persons-suspected-or Accessed 5 July 2020.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–8.
Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–33.
Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233.
Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–6.
Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209.
Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–77.
Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–32.
Schaller T, Hirschbuhl K, Burkhardt K, Braun G, Trepel M, Markl B, et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–20.
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–8.
Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–4.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2.
Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–14.
Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–40.
Edler C, Schroder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Leg Med. 2020;134:1275–84.
Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–61.
Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. E Clin Med. 2020;24:100434.
Sauter JL, Baine MK, Butnor KJ, Buonocore DJ, Chang JC, Jungbluth AA, et al. Insights into pathogenesis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology. 2020;77:915–25.
Grimes Z, Bryce C, Sordillo EM, Gordon RE, Reidy J, Paniz Mondolfi AE, et al. Fatal pulmonary yhromboembolism in SARS-CoV-2-infection. Cardiovasc Pathol. 2020;48:107227.
Schaefer IM, Padera RF, Solomon IH, Kanjilal S, Hammer MM, Hornick JL, et al. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod Pathol. 2020;33:2104–14.
Duarte-Neto AN, Monteiro RAA, da Silva LFF, Malheiros D, de Oliveira EP, Theodoro-Filho J, et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–97.
Yan L, Mir M, Sanchez P, Beg M, Peters J, Enriquez O, et al. COVID-19 in a hispanic woman. Arch Pathol Lab Med. 2020;144:1041–7.
Fitzek A, Sperhake J, Edler C, Schroder AS, Heinemann A, Heinrich F et al. Evidence for systematic autopsies in COVID-19 positive deceased: case report of the first German investigated COVID-19 death. Rechtsmedizin. 2020;1–6. https://doi.org/10.1007/s00194-020-00401-4.
Autopsy Covid-. Electronic address: anapat.hrc@salud.madrid.org. The first COVID-19 autopsy in Spain performed during the early stages of the pandemic. Rev Esp Patol. 2020;53:182–7.
Pernazza A, Mancini M, Rullo E, Bassi M, De Giacomo T, Rocca CD, et al. Early histologic findings of pulmonary SARS-CoV-2 infection detected in a surgical specimen. Virchows Arch. 2020;477:743–8.
Zhang H, Wang CY, Zhou P, Yue H, Du R. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;173:324.
Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33:2156–68.
Bosmuller H, Traxler S, Bitzer M, Haberle H, Raiser W, Nann D, et al. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020;477:349–57.
Youd E, Moore L. COVID-19 autopsy in people who died in community settings: the first series. J Clin Pathol. 2020;73:840–4.
Pritt BS, Aubry MC. Histopathology of viral infections of the lung. Semin Diagn Pathol. 2017;34:510–7.
Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah N, Al Ajlan A, et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection—clinicopathological and ultrastructural study. Histopathology. 2018;72:516–24.
Franks TJ, Chong PY, Chui P, Galvin JR, Lourens RM, Reid AH, et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34:743–8.
Dittmayer C, Meinhardt J, Radbruch H, Radke J, Heppner BI, Heppner FL, et al. Why misinterpretation of electron micrographs in SARS-CoV-2-infected tissue goes viral. Lancet. 2020;396:e64–e65.
Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, et al. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020;26:2005–15.
Fox SE, Li G, Akmatbekov A, Harbert JL, Lameira FS, Brown JQ, et al. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142:1123–5.
Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–35.
Lindner D, Fitzek A, Brauninger H, Aleshcheva G, Edler C, Meissner K, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–5.
Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–5.
Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7:2440–7.
Wenzel P, Kopp S, Gobel S, Jansen T, Geyer M, Hahn F, et al. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res. 2020;116:1661–3.
Yadav H, Kor DJ. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2015;309:L915–923.
Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:2158–67.
Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–27.
Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing gGlomerulopathy in a patient With COVID-19. Kidney Int Rep. 2020;5:935–9.
Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–31.
Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:1959–68.
Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Nickolas T, et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5:940–5.
Magoon S, Bichu P, Malhotra V, Alhashimi F, Hu Y, Khanna S, et al. COVID-19-related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. 2020;2:488–92.
Rossi GM, Delsante M, Pilato FP, Gnetti L, Gabrielli L, Rossini G, et al. Kidney biopsy findings in a critically Ill COVID-19 patient with dialysis-dependent acute kidney injury: a case against “SARS-CoV-2 nephropathy”. Kidney Int Rep. 2020;5:1100–5.
Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, et al. COVID-19-associated -kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31:1948–58.
Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR. COVID-19-associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. 2020;2:493–7.
Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J Am Soc Nephrol. 2020;31:1688–95.
Zhang H, Li HB, Lyu JR, Lei XM, Li W, Wu G, et al. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis. 2020;96:19–24.
Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001.
Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–73.
Song Y, Liu P, Shi XL, Chu YL, Zhang J, Xia J, et al. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69:1143–4.
Kant R, Chandra L, Antony MA, Verma V. Case of COVID-19 presenting with gastrointestinal symptoms. World J Virol. 2020;9:1–4.
Adachi T, Chong JM, Nakajima N, Sano M, Yamazaki J, Miyamoto I, et al. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19, Japan. Emerg Infect Dis. 2020;26.
Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, et al. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am J Clin Pathol. 2020;154:190–200.
Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC. et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–7.
Carnevale S, Beretta P, Morbini P. Direct endothelial damage and vasculitis due to SARS-CoV-2 in small bowel submucosa of COVID-19 patient with diarrhea. J Med Virol. 2020. https://doi.org/10.1002/jmv.26119.
Ignat M, Philouze G, Aussenac-Belle L, Faucher V, Collange O, Mutter D. et al. Small bowel ischemia and SARS-CoV-2 infection: an underdiagnosed distinct clinical entity. Surgery. 2020;168:14–6.
Norsa L, Valle C, Morotti D, Bonaffini PA, Indriolo A, Sonzogni A. Intestinal ischemia in the COVID-19 era. Dig Liver Dis. 2020;52:1090–1.
Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–6.
Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–16.
Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–30.
Craver R, Huber S, Sandomirsky M, McKenna D, Schieffelin J, Finger L. Fatal eosinophilic myocarditis in a healthy 17-year-old male with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2c). Fetal Pediatr Pathol. 2020;39:263–8.
Aguiar D, Lobrinus JA, Schibler M, Fracasso T, Lardi C. Inside the lungs of COVID-19 disease. Int J Leg Med. 2020;134:1271–4.
Suess C, Hausmann R. Gross and histopathological pulmonary findings in a COVID-19 associated death during self-isolation. Int J Leg Med. 2020;134:1285–90.
Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020;159:367–70.
Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18:2128–30 e2122.
Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev. 2020; 41.
Forouzesh M, Rahimi A, Valizadeh R, Dadashzadeh N, Mirzazadeh A. Clinical display, diagnostics and genetic implication of novel Coronavirus (COVID-19) epidemic. Eur Rev Med Pharm Sci. 2020;24:4607–15.
Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, et al. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members. Pancreatology. 2020;20:665–7.
Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253.
Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19). Front Immunol. 2020;11:827.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9.
Xu X, Chang XN, Pan HX, Su H, Huang B, Yang M. et al. Pathological changes of the spleen in ten patients with coronavirus disease 2019(COVID-19) by postmortem needle autopsy. Zhonghua Bing Li Xue Za Zhi. 2020;49:576–82.
Hopfer H, Herzig MC, Gosert R, Menter T, Hench J, Tzankov A et al. Hunting coronavirus by transmission electron microscopy - a guide to SARS-CoV-2-associated ultrastructural pathology in COVID-19 tissues. Histopathology. 2020. https://doi.org/10.1111/his.14264.
Zhang Y, Cao W, Xiao M, Li YJ, Yang Y, Zhao J. et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006
Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–e213.
Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, Pindado-Ortega C, Prieto-Barrios M, Jimenez-Cauhe J. Comment on: Cutaneous manifestations in COVID-19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol. 2020;34:e252–e254.
Young S, Fernandez AP. Skin manifestations of COVID-19. Cleve Clin J Med. 2020. https://doi.org/10.3949/ccjm.87a.ccc031.
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13.
Gianotti R, Veraldi S, Recalcati S, Cusini M, Ghislanzoni M, Boggio F, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
Llamas-Velasco M, Munoz-Hernandez P, Lazaro-Gonzalez J, Reolid-Perez A, Abad-Santamaria B, Fraga J, et al. Thrombotic occlusive vasculopathy in a skin biopsy from a livedoid lesion of a patient with COVID-19. Br J Dermatol. 2020;183:591–3.
Kolivras A, Dehavay F, Delplace D, Feoli F, Meiers I, Milone L, et al. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489–92.
Ahouach B, Harent S, Ullmer A, Martres P, Begon E, Blum L, et al. Cutaneous lesions in a patient with COVID-19: are they related? Br J Dermatol. 2020;183:e31.
Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:e244–e245.
Marzano AV, Genovese G, Fabbrocini G, Pigatto P, Monfrecola G, Piraccini BM, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83:280–5.
Diotallevi F, Campanati A, Bianchelli T, Bobyr I, Luchetti MM, Marconi B, et al. Skin involvement in SARS-CoV-2 infection: case series. J Med Virol. 2020;92:2332–4.
Estebanez A, Perez-Santiago L, Silva E, Guillen-Climent S, Garcia-Vazquez A, Ramon MD. Cutaneous manifestations in COVID-19: a new contribution. J Eur Acad Dermatol Venereol. 2020;34:e250–e251.
Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75–81.
Sohier P, Matar S, Meritet JF, Laurent-Roussel S, Dupin N, Aractingi S. Histopathological features of Chilblain-like lesions developing in the setting of the COVID-19 pandemic. Arch Pathol Lab Med. 2020. https://doi.org/10.5858/arpa.2020-0613-SA.
Kantonen J, Mahzabin S, Mayranpaa MI, Tynninen O, Paetau A, Andersson N, et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020;30:1012–6.
Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological features of Covid-19. N Engl J Med. 2020;383:989–92.
von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395:e109.
Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6.
Matschke J, Lutgehetmann M, Hagel C, Sperhake JP, Schroder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–29.
Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte-Neto AN, Soares Gomes-Gouvea M, Viu Degaspare N, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4:790–4.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–82.
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33.
Patel SR, Karmpaliotis D, Ayas NT, Mark EJ, Wain J, Thompson BT, et al. The role of open-lung biopsy in ARDS. Chest. 2004;125:197–202.
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49.
Libby LJ, Gelbman BD, Altorki NK, Christos PJ, Libby DM. Surgical lung biopsy in adult respiratory distress syndrome: a meta-analysis. Ann Thorac Surg. 2014;98:1254–60.
Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage-the role of oxygen, shock, and related factors. A review. Am J Pathol. 1976;85:209–28.
Hughes KT, Beasley MB. Pulmonary manifestations of acute lung injury: more than just diffuse alveolar damage. Arch Pathol Lab Med. 2017;141:916–22.
Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5:1185–91.
Andrews CP, Coalson JJ, Smith JD, Johanson WG Jr. Diagnosis of nosocomial bacterial pneumonia in acute, diffuse lung injury. Chest. 1981;80:254–8.
Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1985;132:485–9.
Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–32.
Suchyta MR, Orme JF Jr, Morris AH. The changing face of organ failure in ARDS. Chest. 2003;124:1871–9.
Del Sorbo L, Slutsky AS. Acute respiratory distress syndrome and multiple organ failure. Curr Opin Crit Care. 2011;17:1–6.
Hariri L, Hardin CC. Covid-19, angiogenesis, and ARDS endotypes. N Engl J Med. 2020;383:182–3.
Cattaneo M, Bertinato EM, Birocchi S, Brizio C, Malavolta D, Manzoni M, et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120:1230–2.
Deshpande C. Thromboembolic findings in COVID-19 autopsies: pulmonary thrombosis or embolism? Ann Intern Med. 2020;173:394–5.
Lax SF, Skok K, Trauner M. et al. Pulmonary arterial thrombosis as an important complication of COVID-19 pulmonary disease: letter to the editor. Virchows Arch. 2020;477:467–8.
Beasley MB. The pathologist’s approach to acute lung injury. Arch Pathol Lab Med. 2010;134:719–27.
Leslie S, Gill IS, de Castro Abreu AL, Rahmanuddin S, Gill KS, Nguyen M, et al. Renal tumor contact surface area: a novel parameter for predicting complexity and outcomes of partial nephrectomy. Eur Urol. 2014;66:884–93.
Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–66.
Konopka KE, Nguyen T, Jentzen JM, Rayes O, Schmidt CJ, Wilson AM, et al. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Histopathology. 2020;77:570–8.
Hariri LP, North CM, Shih AR, Israel RA, Maley JH, Villalba JA, et al. Lung histopathology in coronavirus disease 2019 as compared with severe acute respiratory sydrome and H1N1 influenza: a systematic review. Chest. 2021;159:73–84.
Chang JC. Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: based on hemostatic theory and endothelial molecular pathogenesis. Clin Appl Thromb Hemost. 2019;25:1076029619887437.
Wang T, Liu Z, Wang Z, Duan M, Li G, Wang S, et al. Thrombocytopenia is associated with acute respiratory distress syndrome mortality: an international study. PLoS ONE. 2014;9:e94124.
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–38.
Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–5.
Nicholas Cossey L, Larsen CP, Liapis H. Collapsing glomerulopathy: a 30-year perspective and single, large center experience. Clin Kidney J. 2017;10:443–9.
Markowitz GS, Nasr SH, Stokes MB, D’Agati VD. Treatment with IFN-{alpha}, -{beta}, or -{gamma} is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:607–15.
Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol. 2001;12:1164–72.
Larsen CP, Beggs ML, Walker PD, Saeed M, Ambruzs JM, Messias NC. Histopathologic effect of APOL1 risk alleles in PLA2R-associated membranous glomerulopathy. Am J Kidney Dis. 2014;64:161–3.
Larsen CP, Beggs ML, Saeed M, Walker PD. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol. 2013;24:722–5.
Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–37.
McNicholas BA, Nelson PJ. Immunity unmasks APOL1 in collapsing glomerulopathy. Kidney Int. 2015;87:270–2.
Couser WG, Johnson RJ. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int. 2014;86:905–14.
Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: a comprehensive review. World J Gastroenterol. 2020;26:2323–32.
Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231–40.
Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92:1491–4.
Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis. 2020;63:682–9.
Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized With COVID-19 infection. J Am Coll Cardiol. 2020;76:533–46.
Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76:2043–55.
Grun S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–15.
Greulich S, Seitz A, Muller KAL, Grun S, Ong P, Ebadi N, et al. Predictors of mortality in patients with biopsy-proven viral myocarditis: 10-year outcome data. J Am Heart Assoc. 2020;9:e015351.
Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24:491.
Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–8.
Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–91.
Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296:E119–E120.
Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–6.
Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–20.
Klejtman T. Skin and COVID-19. J Med Vasc. 2020;45:175–6.
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–95.
Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–8.
Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, et al. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146.
Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–8.
Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–69.
Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–6.
European Centre for Disease Prevention and Control. Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS -CoV-2 infection in children. 2020. https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment. Accessed 30 Oct 2020.
Centers for Disease Control and Prevention. Emergency preparedness and response: multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Health advisory. 2020. https://emergency.cdc.gov/han/2020/han00432.asp. Accessed 22 Aug 2020.
Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–58.
Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–46.
Davies P, Evans C, Kanthimathinathan HK, Lillie J, Brierley J, Waters G, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4:669–77.
Rowley AH, Baker SC, Arrollo D, Gruen LJ, Bodnar T, Innocentini N, et al. A protein epitope targeted by the antibody response to Kawasaki disease. J Infect Dis. 2020;222:158–68.
Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20:453–4.
Mahase E. Covid-19: Cases of inflammatory syndrome in children surge after urgent alert. BMJ. 2020;369:m1990.
Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–36.
Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–81 e967.
Sokolovsky S, Soni P, Hoffman T, Kahn P, Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am J Emerg Med. 2021;39:253 e251–253 e252.
Shaigany S, Gnirke M, Guttmann A, Chong H, Meehan S, Raabe V, et al. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet. 2020;396:e8–e10.
Lidder AK, Pandit SA, Lazzaro DR. An adult with COVID-19 kawasaki-like syndrome and ocular manifestations. Am J Ophthalmol Case Rep. 2020;20:100875.
Miller SE, Brealey JK. Visualization of putative coronavirus in kidney. Kidney Int. 2020;98:231–2.
Miller SE, Goldsmith CS. Caution in identifying coronaviruses by electron microscopy. J Am Soc Nephrol. 2020;31:2223–4.
Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395:e99.
Calomeni E, Satoskar A, Ayoub I, Brodsky S, Rovin BH, Nadasdy T. Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int. 2020;98:233–4.
Scholkmann F, Nicholls J. Pulmonary vascular pathology in Covid-19. N Engl J Med. 2020;383:887–8.
Roufosse C, Curtis E, Moran L, Hollinshead M, Cook T, Hanley B, et al. Electron microscopic investigations in COVID-19: not all crowns are coronas. Kidney Int. 2020;98:505–6.
Smith KD, Akilesh S, Alpers CE, Nicosia RF. Am I a coronavirus? Kidney Int. 2020;98:506–7.
Bangash MN, Patel JM, Parekh D, Murphy N, Brown RM, Elsharkawy AM, et al. SARS-CoV-2: is the liver merely a bystander to severe disease? J Hepatol. 2020;73:995–6.
Bullock HA, Goldsmith CS, Zaki SR, Martines RB, Miller SE. Difficulties in differentiating coronaviruses from subcellular structures in human tissues by electron microscopy. Emerg Infect Dis. 2021;27:1023–31.
Author information
Authors and Affiliations
Contributions
GAG and SC performed study concept, design, and development of methodology; GAG, SC and MEK provided acquisition, analysis, and interpretation of data, writing, review, and revision; SEM, RE, JJ, and GS provided review and revision; AM and GE provided technical and material support. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
GE is a microscopy and imaging specialist at Hunt Optics & Imaging, Inc, Pittsburgh, PA. All other authors have no relevant disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Caramaschi, S., Kapp, M.E., Miller, S.E. et al. Histopathological findings and clinicopathologic correlation in COVID-19: a systematic review. Mod Pathol 34, 1614–1633 (2021). https://doi.org/10.1038/s41379-021-00814-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00814-w
This article is cited by
-
Artificial intelligence-based analysis of the spatial distribution of abnormal computed tomography patterns in SARS-CoV-2 pneumonia: association with disease severity
Respiratory Research (2024)
-
Cardiovascular complications of diabetes: role of non-coding RNAs in the crosstalk between immune and cardiovascular systems
Cardiovascular Diabetology (2023)
-
Differential activation of programmed cell death in patients with severe SARS-CoV-2 infection
Cell Death Discovery (2023)
-
Virologic Studies in COVID-Positive Donors
Current Transplantation Reports (2023)
-
Liver alterations and detection of SARS-CoV-2 RNA and proteins in COVID-19 autopsies
GeroScience (2023)