Abstract
Microglandular adenosis (MGA)-related lesions, including atypical MGA (AMGA) and carcinoma involving MGA (C-MGA), are characterized by epithelial atypia, negative hormone receptors, and HER2 status, and can mimic invasive triple negative breast cancer (TNBC) in core needle biopsies (CNB) resulting in selection for treatment with neoadjuvant chemotherapy (NAC). We identified 12 cases of AMGA and/or C-MGA in post-NAC excision specimens (EXC) and analyzed their morphologic and immunohistochemical (IHC) features. All CNBs were initially diagnosed as containing TNBC. Upon re-review, TNBC was confirmed in nine cases. In three CNBs AMGA and/or C-MGA had been interpreted as TNBC. AMGA was initially recognized in only one case but AMGA and/or C-MGA were present in an additional nine CNBs. At EXC, no residual TNBC was present in 5 of 9 EXCs and all 12 cases showed residual AMGA and/or C-MGA. Similar to conventional MGA, AMGA, and C-MGA were positive for S-100, laminin and collagen IV and negative for calponin and p63. Following NAC, these lesions retained their typical staining pattern despite acquiring treatment-related morphologic alterations, most notably of which were areas of single cell growth pattern seen in eight EXCs. This study is the first to report the effects of NAC on AMGA and C-MGA. Our data showed no response of the AMGA and/or C-MGA following NAC in contrast to the high response rate of conventional TNBC. In particular, the infiltrative single cell pattern of post-NAC MGA-related lesions closely mimicked residual TNBC. The persistence of AMGA and C-MGA following NAC supports the notion that these lesions are distinct from conventional TNBC. Our findings also highlight the challenges in recognizing AMGA and C-MGA in CNBs which may lead to unwarranted treatment with NAC in the absence of conventional TNBC.
Similar content being viewed by others
Introduction
Microglandular adenosis (MGA) is a rare, unusual proliferation in which small glands haphazardly permeate the breast. While delineated by a thin basement membrane, the glands do not possess myoepithelial cells. The cells of MGA are estrogen (ER) and progesterone receptor (PR) negative, do not show human epidermal growth factor receptor-2 (HER2) overexpression and are consistently positive for S-100 [1]. In addition, concurrent invasive carcinoma may be present in up to 27% of cases [2,3,4]. Rosenblum et al. [3] were the first to propose that carcinomas may develop from MGA. In a series of eight cases, the authors found identifiable MGA intimately associated with carcinoma in all patients and noted transitional patterns of MGA exhibiting atypia. Atypical MGA (AMGA) has the growth pattern of typical MGA but the cells show nuclear and architectural atypia. When the proliferations become expansile, obliterate glandular lumens, show severe cytologic atypia with increased apoptotic and mitotic figures and lack a stromal desmoplastic reaction typical of NOS type invasive ductal carcinoma while still retaining the distinct growth pattern of MGA, the diagnosis of carcinoma involving MGA (C-MGA) can be rendered [2]. It should be noted, however, that AMGA and C-MGA represent a spectrum of morphologic changes from moderate to marked atypia and as a result the two entities can be difficult to delineate. Nevertheless, the diagnosis of C-MGA is best considered as equivalent to ductal carcinoma in situ (DCIS).
The seemingly infiltrative growth pattern and marked atypia of these MGA-related lesions along with the absence of myoepithelial cells mimic invasive carcinoma, making the distinction in core needle biopsy (CNB) specimens especially challenging. Additionally, because nearly all AMGA and C-MGA are negative for hormone receptors and HER2, there is potential for misdiagnosis as triple negative invasive breast carcinoma (TNBC). As most patients with TNBC are now offered neoadjuvant chemotherapy (NAC), this may result in overtreatment when systemic therapy is not indicated. We have observed cases from both our consultation service and in house breast service of AMGA and C-MGA that led to diagnostic difficulties.
This study aims to highlight the morphologic and immunohistochemical (IHC) features of AMGA and C-MGA in material obtained by CNB and post-NAC excision/mastectomy (EXC) specimens and to raise awareness of these lesions to prevent diagnostic pitfalls and consequently patient overtreatment.
Results
Between 2011 and 2019, 12 cases from 12 women were identified which met the above inclusion criteria. Ten CNB had been obtained at other centers and the slides were reviewed as departmental consultation cases when the patient transferred care to our center. Two CNBs had been performed in-house (cases 11 and 12). Ten EXCs were performed in-house and slides of 2 EXCs were reviewed in consultation (cases 6 and 9).
Clinical data
The average patient age was 46 years (range 29–77) (Table 1). Imaging findings were available for 11 cases and all showed an irregular mass with a median size of 2.33 cm (range 0.9–3.5). Physical examination revealed a palpable mass in eight patients, an area of fullness without a distinct mass in one and no specific findings in a patient who had a sub-centimeter mass on mammogram. All patients received NAC: ten patients received anthracycline and taxanes (ACT), one was treated with CMF, and in one consultation case the regimen was unspecified. Following NAC, seven patients underwent mastectomy and five had breast conserving surgery. Eight patients had sentinel lymph node biopsy, two had axillary lymph node dissection, one had no formal axillary lymph node dissection but a few axillary lymph nodes were found with the mastectomy specimen and one had no axillary lymph node sampling. According to available information, six patients received radiation therapy following surgery and five did not.
Pre-NAC CNB pathologic findings
The pathologic features of the study cohort are summarized in Table 2. Out of the ten CNB consultation cases, only one CNB was initially submitted with a diagnosis indicating the presence of MGA of any type (case 4), which was confirmed during consultation review. In this case, MGA with areas transitioning into AMGA were identified at the periphery of the TNBC. Six of the remaining CNB consultation cases were confirmed to contain either AMGA or C-MGA following morphologic re-review: three CNBs showed both AMGA and C-MGA, two CNBs showed AMGA and one showed only C-MGA. Three CNBs also showed small foci of MGA (Fig. 1). In three CNBs (cases 6-8), no MGA, AMGA or C-MGA was present; in one of these three CNBs focal rounded nests were noted at the edge of the core biopsy that were suspicious for AMGA but could not be definitively confirmed in the available submitted material. The two CNBs done in-house (cases 11 and 12) were originally diagnosed as showing TNBC only, however, following review for this study AMGA and C-MGA were also identified. Conventional DCIS was present in 3 of 12 CNBs.
All 12 CNBs were originally reported as having TNBC. After morphologic re-review for this study, 9 were confirmed to contain TNBC. All invasive carcinomas were poorly differentiated, consisting of solid nests of infiltrating tumor cells without gland formation, moderate to marked nuclear atypia and scattered mitoses. Three cases showed focal myxoid stromal changes and matrix production consistent with metaplastic carcinoma. Invasive tumors were surrounded by desmoplastic stromal reactions and/or dense lymphocytic infiltrates. Definite TNBC could not be confirmed in three CNBs (cases 1–3), all consultation cases. In these cases, the foci originally interpreted as invasive carcinoma in fact represented foci of AMGA and/or C-MGA based on their distinct architectural growth pattern and lack of stromal reaction (Fig. 2). While no IHC stained slides were submitted for the consultation, in case 1 the presence of adjacent typical MGA aided in the diagnosis of AMGA/C-MGA. Cases 2 and 3 were both reported to be S-100 positive and showed absence of myoepithelial staining with p63 and calponin on submitted IHC slides. No basement membrane stains were performed on the CNB material of these three cases at the submitting institutions. For the remaining CNB with available IHC, immunoreactivity for S-100 protein and laminin/collagen IV aided in confirmation of the presence of AMGA and C-MGA.
Post-NAC EXC pathologic findings
At excision, all 12 cases showed either AMGA or C-MGA. One EXC (case 7), which did not show evidence of AMGA or C-MGA in the submitted CNB, also showed MGA. The extent of MGA, AMGA, and/or C-MGA was measured as the largest contiguous microscopic span on a single representative tissue section. The median span was 2.2 cm (range 0.5–3.0). The three cases with no confirmed TNBC on CNB showed no invasive carcinoma at EXC and no residual TNBC was seen at EXC in five of the nine cases with confirmed TNBC on CNB; all had persistent AMGA and/or C-MGA (Fig. 3). Residual TNBC was seen in four EXCs (Fig. 4). The residual TNBC measured 0.2, 0.3, 3.0 and 6.5 cm, microscopically. DCIS was seen in two EXCs without residual invasive tumor. Residual carcinoma was identified in a background of tumor bed changes, consisting of stromal fibrosis, histiocytic aggregates, and chronic inflammation. Metastatic carcinoma involving axillary lymph nodes was identified in two patients who underwent axillary lymph node dissection. For one EXC (case 6), a consultation case, the axillary contents were entirely submitted and only one lymph node was identified.
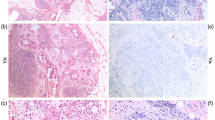
A Low power view of tissue section showing biopsy site changes in upper left, treatment related changes without residual invasive carcinoma in center, and persistent AMGA/C-MGA in lower right. Inset shows higher power of AMGA/C-MGA exhibiting single cell pattern (H&E, 20X; inset H&E, 50X); B Absence of myoepithelial cells around AMGA/C-MGA (ADH5, 100X); C Residual AMGA/C-MGA with positive nuclear staining with S-100 protein (100X); Preservation of basement membrane surrounding AMGA/C-MGA (D collagen IV, 100X; E laminin, 100X).
Residual poorly differentiated invasive carcinoma with surrounding inflammatory infiltrate and stromal reactive changes and extensive residual AMGA/C-MGA present as small glands, interconnected chains and single cells with an infiltrative pattern (A H&E, 10X; B H&E, 100X; C H&E, 50X); D Poorly differentiated invasive carcinoma with desmoplastic stromal reaction (H&E, 50X); Absence of basement membrane surrounding residual invasive carcinoma (E collagen IV, 50X; F laminin, 50X); G AMGA/C-MGA with infiltrative single cell pattern without stromal reactive changes (H&E, 100X); Preservation of basement membrane around AMGA/C-MGA (H collagen IV, 50X; I laminin, 50X); Absence of myoepithelial cells around AMGA/C-MGA (J calponin, 50X; K p63, 50X); L AMGA/C-MGA showing positive nuclear staining with S-100 protein (50X).
Following NAC, AMGA, and C-MGA showed morphologic alterations consisting of increased pleomorphism with scattered anaplastic nuclear features, increased cytoplasm with vacuolization and reduced size of glands (Fig. 5). Prominent coarse eosinophilic cytoplasmic granules were identified in AMGA and C-MGA in four EXCs. These granulated cells, where present, comprised a small minority of the neoplastic cells making up the residual AMGA/C-MGA in the EXCs. Small and faint eosinophilic cytoplasmic granules were also focally identified in MGA in all three CNBs in which it was present and in adjacent AMGA in those CNBs. Eight EXCs revealed areas of AMGA and C-MGA which showed scattered single cell and small cell clusters infiltrating through predominantly densely collagenous stroma and, in areas, into adipose tissue, devoid of desmoplastic reaction. The overall growth was in a pattern reminiscent of the underlying MGA, having a haphazard or alveolar type growth and without compression or alteration of preexisting ducts and lobules (Figs. 3–5).
Immunohistochemical findings
All invasive carcinomas, MGA, AMGA, and C-MGA were ER, PR, and HER2 IHC negative. S-100 protein, performed in 11 cases, was consistently positive in MGA, AMGA, C-MGA and in associated TNBC. The AMGA and C-MGA showed delineation of the basement membrane with laminin and collagen IV stains in nine cases and ten cases, respectively. Basement membrane staining pattern was heterogeneous within and between cases with both strong, dark circumferential staining and weak, faint patchy expression. In cases with TNBC, laminin and collagen IV stains were predominantly negative around the invasive tumor, however focal disrupted staining was identified in some cases. Calponin and p63 stains were negative around AMGA/C-MGA in five and six cases, respectively. Additionally, calponin and p63 were negative around TNBC in cases in which it was present. The AMGA and C-MGA growing as single cells also stained positively for S-100 and demonstrated circumferential staining with laminin and collagen IV. No myoepithelial cell staining was present.
Outcomes
Follow-up data was available for 11 patients (Table 1). The median time of follow-up was 16 months (range 1–45). One patient (case 11) developed a chest wall recurrence and a lung metastasis 19 months after surgery. This patient was found to have TNBC and AMGA on her pre-NAC CNB. Post-NAC she had 3.0 cm residual TNBC, AMGA and metastatic carcinoma in two lymph nodes. She received post-mastectomy radiation. At present, the patient is alive with widely metastatic disease (bone, liver, pleura, brain and meninges). The remaining patients show no evidence of disease at last follow-up.
Discussion
This series is the first to examine the effects of NAC on AMGA and C-MGA. Although we observed treatment related morphologic alterations, there appeared to be minimal response of AMGA and C-MGA to NAC, in contrast to the high response rate of conventional TNBC. While more than half of the cases in this study showed complete pathologic resolution of the invasive carcinoma, all cases showed persistence of extensive AMGA and/or C-MGA.
In this data set, 75% (9/12) of cases were confirmed to contain AMGA and/or C-MGA on CNB, however AMGA was only initially identified in one case. More importantly, 25% (3/12) of CNBs had only AMGA and/or C-MGA, which were initially interpreted as TNBC. These findings highlight the challenges in recognizing AMGA/C-MGA in CNB. The triple negative phenotype, degree of cytologic and architectural atypia, and lack of peripheral myoepithelial cells can make differentiation from invasive carcinoma difficult, especially in CNB specimens. This is further complicated by the infiltrative nature of these lesions, which closely mimic invasive carcinoma. However, bona fide invasive carcinoma arising in this setting is characterized by expansile and coalescent growth, desmoplastic reaction and destruction of normal architecture, while the overall appearance of AMGA/C-MGA still reflects the underlying pattern of MGA which characteristically shows haphazard irregular glands that surround but do not compress or alter preexisting ducts and lobules. AMGA/C-MGA may show a multinodular or alveolar growth pattern and may appear solid at low power magnification but on closer evaluation individual acini packed together can be appreciated. The identification of adjacent typical MGA can also suggest the diagnosis however typical MGA may not be present in limited CNB specimens. As illustrated in this study, while pathologists should remember that invasive carcinomas occur much more frequently than MGA, being more familiar with the morphologic pattern of MGA will aid in the recognition of AMGA/C-MGA. Desmoplasia can be a key feature in differentiation of AMGA/C-MGA from TNBC as it is absent in the former and variably prominent in the latter. The IHC profile of IDC associated with AMGA/C-MGA to a large extent resembles that of MGA itself (cytokeratin and S100 positive, negative for myoepithelial cell markers) however, as our data confirms, basement membrane material will be preserved in AMGA/C-MGA and disrupted or completely absent in TNBC.
The distinction between IDC and AMGA/C-MGA can be even more challenging post-NAC due to treatment related morphologic alterations, including anaplastic cytologic features with bizarre nuclear figures and increased, vacuolated cytoplasm and prominent eosinophilic granules. While we identified some fine, cytoplasmic eosinophilic granules in areas of MGA pre-NAC, the granularity was coarse and more pronounced following therapy. The clinical significance of this finding is unclear with some suggesting it simply represents a degenerative phenomenon related to cell injury and death, as these large eosinophilic globules have been identified in high numbers in those tumors which had received prior treatment (radiation or chemotherapy) [5]. Whilst the presence of cytoplasmic granules typically raises the differential diagnosis between MGA and acinic cell carcinoma (ACC), Huo et al. [6] argue against the finding of prominent eosinophilic granules as diagnostic for one or the other, concluding instead that they most likely represent a nonspecific, metaplastic process. None of the lesions in our series showed an overall morphology of ACC. The cells of ACC have variably basophilic and eosinophilic granular cytoplasm, imparting a variegated appearance, unlike the cells in our cohort which showed eosinophilic to clear cytoplasm with focal eosinophilic granules, making up only a small subset of the neoplastic cell population [7]. It has been proposed that expression of lysozyme and α1-antichymotrypsin, markers of serous differentiation, can be useful in the confirmation of ACC however diffuse expression of these enzymes have been observed in MGA, AMGA, secretory carcinoma, cystic hypersecretory carcinoma, normal lactating breast and apocrine metaplastic cells, and expression has even been reported in many common breast cancers as well [8,9,10,11,12]. It is our recommendation that these IHC assays should be used only after identifying histologic features strongly suggestive of ACC to aid in confirmation of that diagnosis. Likewise, although laminin and collagen IV expression have been shown to be typically absent around the neoplastic glands of ACC there are reports of circumferential staining in some of these tumors [7]. In contrast, the preservation of the basement membrane is well documented and is characteristic in AMGA and C-MGA [7, 8].
Most notable was our observation of a recurrent scattered single cell and small cell cluster pattern, seen in 67% (8/12) of excisions, reminiscent of the appearance of many residual invasive carcinomas post NAC. A similar partial pathologic response of scattered residual tumor cells has been reported in up to 40% of post-NAC cases and, to complicate matters further, residual invasive carcinoma has also been described as being embedded in a densely fibrotic tumor bed without significant desmoplasia [13]. The presence of chemotherapy induced histomorphologic changes in the stroma and surrounding non-neoplastic breast tissue can make the identification of reactive stromal changes to an invasive component even more difficult. In these instances, IHC can be a useful adjunct to diagnosis as again we identified consistent preservation of basement membrane, highlighted by laminin and collagen IV, with an accompanying lack of myoepithelial cells surrounding AMGA/C-MGA.
Invasion is fundamentally defined as the breach of the basement membrane, and not just loss of myoepithelial cells [14]. While some invasive carcinomas such as well differentiated cutaneous squamous cell carcinoma and basal cell carcinomas have been reported to display a continuous basement membrane [15], studies of breast neoplasia have repeatedly observed a gradual loss of basement membrane, as demonstrated by IHC studies, with increasing degree of tumor dedifferentiation. Thin, but often fragmented and discontinuous, basement membrane has been seen in well differentiated tumors, including tubular carcinomas, in contrast to its consistent absence of staining in poorly differentiated tumors [16,17,18,19]. In addition, continuous basement membrane staining has been identified surrounding benign and in situ breast lesions, supporting the notion that the absence of basement membrane indicates stromal invasion [20].
The invasive carcinomas arising in association with MGA-related lesions are reported to be poorly differentiated and triple negative in the majority of cases (between 83% [2] and 100% [21, 22] of cases and 75% [21] to 100% [2, 22, 23], respectively). These tumors also often show metaplastic features, particularly chondromyxoid matrix production, observed with varying frequency in almost all reported series [2, 3, 11, 21, 23]. It should be noted that less aggressive triple negative tumor types, including adenoid cystic carcinoma and ACC, have also been seen in association with MGA-related lesions [24, 25]. In our cohort, all cases with invasive carcinoma were poorly differentiated TNBC and in three cases matrix production was identified.
Interestingly, despite most having histopathologic and IHC features usually associated with a poor prognosis, cases of carcinoma arising in association with AMGA/C-MGA have been shown to have a relatively favorable prognosis compared to conventional TNBC. A study from James et al. [21] reported that 12 patients with AMGA and invasive carcinoma were recurrence free after a mean follow up of 54 months and, while one patient did develop a spinal metastasis she was still alive at 98 months. Resetkova et al. [22] detailed the 10-year follow up of a case of C-MGA and invasive carcinoma treated with breast conservation surgery without any additional adjuvant therapy who developed a local breast recurrence which was resected without further incident. Zhong et al. [23] described ten patients without evidence of disease after an average follow up of 27 months. Of note, one patient in this cohort received NAC for a 5.0 cm invasive carcinoma and was alive at 40 months without evidence of disease. Likewise, despite the persistence of extensive AMGA/C-MGA in all cases, our data show good overall outcomes, albeit after a relatively short follow up period. In contrast, patients with usual TNBC have high rates of early metastasis and death from breast cancer within 2–5 years from diagnosis and, when found to have residual disease after NAC, have shorter overall survival than non-TNBC [26]. Taken together these findings strongly support the theory that AMGA and C-MGA are not biologically equivalent to conventional TNBC.
In conclusion, despite its retrospective nature and small sample size due to the relative rarity of these lesions, this study highlights the challenges in recognizing AMGA and C-MGA in CNBs. The triple negative phenotype and morphologic similarity to invasive carcinoma may lead to unwarranted treatment with neoadjuvant therapy. Having only limited CNB material we cannot know the amount of AMGA/C-MGA present pre-treatment, however our data show persistence of extensive residual AMGA/C-MGA after NAC, analogous to post-treatment persistence of DCIS, supporting the hypothesis that AMGA/C-MGA may not be equivalent to conventional TNBC despite its lack of myoepithelial cells. Importantly, the infiltrative single cell growth pattern of residual AMGA and C-MGA, seen in 8 of 12 excisions, could be misdiagnosed for residual invasive carcinoma. With the increasing utilization of NAC for TNBC, familiarity with the morphologic patterns of pre- and post-NAC MGA-related lesions is paramount.
Materials and methods
After obtaining institutional review board approval, we identified post-NAC EXC specimens showing AMGA and/or C-MGA reviewed at our institution through a retrospective search of our pathology department database. Only cases for which the slides of paired EXC and CNB were available for review were included in the study. Demographic and clinical data were recorded. All available hematoxylin and eosin (H&E) and IHC stained slides were reviewed. Only IHC slides, with appropriate positive and negative controls, which were performed during original evaluation of each specimen, either on CNB and/or subsequent EXC, were reviewed. Cases included IHC slides stained at our institution and those stained at outside laboratories.
The distinct components of each case, including MGA, AMGA, C-MGA, conventional DCIS, and TNBC, were categorized and their presence or absence was recorded. Adhering to the reported morphologic features [27,28,29], MGA was defined as small, round glands with generally open lumens containing eosinophilic colloid-like secretions. The glands were composed of a single layer of cytologically bland cells with some containing cytoplasmic eosinophilic granules. The glands were irregularly distributed in a non-lobulocentric pattern, infiltrating between and around uninvolved ducts without altering their structure. AMGA consisted of irregular glands, arranged in a back to back pattern without desmoplasia, exhibiting mild to moderate nuclear pleomorphism and scattered apoptotic and mitotic figures. Lesions with the architectural features of AMGA but exhibiting markedly atypical cells, conspicuous mitoses and no desmoplastic stromal reaction were categorized as C-MGA. All forms of MGA were characterized by a lack of myoepithelial cells and maintenance of a basement membrane layer. DCIS consisted of an intraductal proliferation of neoplastic cells surrounded by a layer of myoepithelial cells. Growth of cohesive carcinoma cells in coalescent irregular nests or cords with associated desmoplastic reaction, without myoepithelial cells and absence or disruption of the basement membrane layer were classified as TNBC.
The American Society of Clinical Oncology/College of American Pathologist [30, 31] scoring criteria were used for determining ER, PR, and HER2 status. Lesional cells were considered positive if they had nuclear and/or cytoplasmic staining for S-100 and circumferential staining for collagen IV and laminin. The presence of myoepithelial cells was highlighted by nuclear staining for p63 and/or cytoplasmic staining for calponin.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Lokuhetty D, White VA, Watanabe R, Cree IA. WHO classification of tumours editorial board. Breast Tumors (Lyon: IARC, 2019).
Khalifeh IM, Albarracin C, Diaz LK, Symmans FW, Edgerton ME, Hwang RF, et al. Clinical, histopathologic, and immunohistochemical features of microglandular adenosis and transition into in situ and invasive carcinoma. Am J Surg Pathol. 2008;32:544–52.
Rosenblum MK, Purrazzella R, Rosen PP. Is microglandular adenosis a precancerous disease? A study of carcinoma arising therein. Am J Surg Pathol. 1986;10:237–45.
Salarieh A, Sneige N. Breast carcinoma arising in microglandular adenosis: a review of the literature. Arch Pathol Lab Med. 2007;131:1397–9.
Papadimitriou JC, Drachenberg CB, Brenner DS, Newkirk C, Trump BF, Silverberg SG. “Thanatosomes”: a unifying morphogenetic concept for tumor hyaline globules related to apoptosis. Hum Pathol. 2000;31:1455–65.
Huo L, Bell D, Qiu H, Sahin A, Wu Y, Sneige N. Paneth cell-like eosinophilic cytoplasmic granules in breast carcinoma. Ann Diagn Pathol. 2011;15:84–92.
Conlon N, Sadri N, Corben AD, Tan LK. Acinic cell carcinoma of breast: morphologic and immunohistochemical review of a rare breast cancer subtype. Hum Pathol. 2016;51:16–24.
Damiani S, Pasquinelli G, Lamovec J, Peterse JL, Eusebi V. Acinic cell carcinoma of the breast: an immunohistochemical and ultrastructural study. Virchows Arch. 2000;437:74–81.
Geyer FC, Berman SH, Marchio C, Burke KA, Guerini-Rocco E, Piscuoglio S, et al. Genetic analysis of microglandular adenosis and acinic cell carcinomas of the breast provides evidence for the existence of a low-grade triple-negative breast neoplasia family. Mod Pathol. 2017;30:69–84.
Hirokawa M, Sugihara K, Sai T, Monobe Y, Kudo H, Sano N, et al. Secretory carcinoma of the breast: a tumour analogous to salivary gland acinic cell carcinoma? Histopathology. 2002;40:223–9.
Koenig C, Dadmanesh F, Bratthauer GL, Tavassoli FA. Carcinoma arising in microglandular adenosis: an immunohistochemical analysis of 20 intraepithelial and invasive neoplasms. Int J Surg Pathol. 2000;8:303–15.
Osako T, Takeuchi K, Horii R, Iwase T, Akiyama F. Secretory carcinoma of the breast and its histopathological mimics: value of markers for differential diagnosis. Histopathology. 2013;63:509–19.
Rajan R, Esteva FJ, Symmans WF. Pathologic changes in breast cancer following neoadjuvant chemotherapy: implications for the assessment of response. Clin Breast Cancer. 2004;5:235–8.
Rakha EA, Miligy IM, Gorringe KL, Toss MS, Greene AR, Fox SB, et al. Invasion in breast lesions: the role of the epithelial-stroma barrier. Histopathology. 2018;72:1075–83.
Rakha EA, Gandhi N, Climent F, van Deurzen CH, Haider SA, Dunk L, et al. Encapsulated papillary carcinoma of the breast: an invasive tumor with excellent prognosis. Am J Surg Pathol. 2011;35:1093–103.
Albrechtsen R, Nielsen M, Wewer U, Engvall E, Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981;41:5076–81.
Raymond WA, Leong AS. Assessment of invasion in breast lesions using antibodies to basement membrane components and myoepithelial cells. Pathology. 1991;23:291–7.
Willebrand D, Bosman FT, de Goeij AF. Patterns of basement membrane deposition in benign and malignant breast tumours. Histopathology. 1986;10:1231–41.
Visscher DW, Sarkar FH, Sakr W, Crissman J. Immunohistologic analysis of invasive phenotype in breast carcinoma. A clinicopathologic study. Pathol Res Pract. 1993;189:867–72.
Esposito NN, Dabbs DJ, Bhargava R. Are encapsulated papillary carcinomas of the breast in situ or invasive? A basement membrane study of 27 cases. Am J Clin Pathol. 2009;131:228–42.
James BA, Cranor ML, Rosen PP. Carcinoma of the breast arising in microglandular adenosis. Am J Clin Pathol. 1993;100:507–13.
Resetkova E, Flanders DJ, Rosen PP. Ten-year follow-up of mammary carcinoma arising in microglandular adenosis treated with breast conservation. Arch Pathol Lab Med. 2003;127:77–80.
Zhong F, Bi R, Yu B, Cheng Y, Xu X, Shui R, et al. Carcinoma arising in microglandular adenosis of the breast: triple negative phenotype with variable morphology. Int J Clin Exp Pathol. 2014;7:6149–56.
Acs G, Simpson JF, Bleiweiss IJ, Hugh J, Reynolds C, Olson S, et al. Microglandular adenosis with transition into adenoid cystic carcinoma of the breast. Am J Surg Pathol. 2003;27:1052–60.
Foschini MP, Eusebi V. Microglandular adenosis of the breast: a deceptive and still mysterious benign lesion. Hum Pathol. 2018;82:1–9.
Geyer FC, Pareja F, Weigelt B, Rakha E, Ellis IO, Schnitt SJ, et al. The spectrum of triple-negative breast disease: high- and low-grade lesions. Am J Pathol. 2017;187:2139–51.
Clement PB, Azzopardi JG. Microglandular adenosis of the breast-a lesion simulating tubular carcinoma. Histopathology. 1983;7:169–80.
Rosen PP. Microglandular adenosis. A benign lesion simulating invasive mammary carcinoma. Am J Surg Pathol. 1983;7:137–44.
Tavassoli FA, Norris HJ. Microglandular adenosis of the breast. A clinicopathologic study of 11 cases with ultrastructural observations. Am J Surg Pathol. 1983;7:731–7.
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95.
Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartless JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice guideline focused update. J Clin Oncol. 2018;36:2105–22.
Acknowledgements
The findings in this study were featured in a platform presentation at the United States and Canadian Academy of Pathology (USCAP) annual conference in Los Angeles, California in March 2020.
Funding
This work was supported in part by a National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30CA008748).
Author information
Authors and Affiliations
Contributions
AG and LT performed study concept and design, analysis and interpretation of data; AG performed investigation and writing, original draft preparation; TD, HW, MM, EB, and LT provided acquisition of data and writing, review and revision of the paper. All authors have read and approved the manuscript and have contributed sufficiently to the project to be included as an author.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grabenstetter, A., Alfonso, T.M.D., Wen, H.Y. et al. Morphologic and immunohistochemical features of carcinoma involving microglandular adenosis of the breast following neoadjuvant chemotherapy. Mod Pathol 34, 1310–1319 (2021). https://doi.org/10.1038/s41379-021-00781-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00781-2