Abstract
Telomerase reverse transcriptase (TERT) promoter mutations have been implicated in urothelial carcinogenesis and are present in 60–80% of conventional and variants of urothelial carcinomas. We investigated the prevalence of TERT promoter mutations in 46 cases of bladder nonurachal adenocarcinoma, 30 cases of urothelial carcinoma with glandular differentiation, 24 cases of nephrogenic adenoma, eight cases of villous adenoma, 31 cases of florid cystitis glandularis, and 20 cases of intestinal metaplasia of the bladder. TERT promoter mutations were detected in 33% of adenocarcinomas of urinary bladder and in 67% of urothelial carcinomas with glandular differentiation. All 30 cases of urothelial carcinoma with glandular differentiation harbored identical TERT promoter mutation in both glandular and urothelial carcinoma components from the same tumor, suggesting a common clonal origin. TERT promoter mutations were absent in nephrogenic adenoma, villous adenoma, florid cystitis glandularis, and intestinal metaplasia of the bladder. TERT promoter mutation analysis may be a useful ancillary study in the differential diagnosis.
Similar content being viewed by others
Introduction
Primary adenocarcinoma of the urinary bladder is an uncommon tumor accounting for 0.5–2% of all bladder malignancies. The diagnosis of primary adenocarcinoma of the urinary bladder could be challenging, especially in small biopsy specimens [1, 2]. Histologically, primary adenocarcinoma manifests as pure adenocarcinoma with various morphologies including enteric, mucinous, or mixed pattern, with or without extracellular mucin [3]. The presence of any urothelial carcinoma component will exclude the diagnosis of primary adenocarcinoma of the urinary bladder.
Mutations in the TERT promoter have been implicated in carcinogenesis of 60–80% of urothelial carcinomas but the molecular pathways for primary adenocarcinoma of the urinary bladder are not well defined [4,5,6,7,8,9]. In this study, we evaluated TERT promoter mutations in 46 cases of primary adenocarcinoma of the urinary bladder and in 30 cases of urothelial carcinoma with glandular differentiation to gain further insight into the molecular carcinogenesis and the possible link to conventional urothelial carcinoma.
Materials and methods
Patients
Specimens from 46 patients with nonurachal adenocarcinoma of the urinary bladder and from 30 patients with urothelial carcinoma with glandular differentiation were retrieved from the archives of participating institutions. The diagnoses of primary adenocarcinoma of the urinary bladder and of urothelial carcinoma with glandular differentiation were based on the criteria in the World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs [3]. All slides were reviewed retrospectively, and the diagnoses were confirmed. None of the patients with primary adenocarcinoma of the urinary bladder had history of prostate cancer or radiation therapy. Additionally, 24 cases of nephrogenic adenoma, eight cases of villous adenoma, 31 cases of florid cystitis glandularis, and 20 cases of intestinal metaplasia of the bladder were included in the TERT promoter mutation analysis.
This research was approved by the Institutional Review Boards of participating institutions in accordance with each Institutional Committee for the Protection of Human Subjects.
High-resolution fluorescence melting curve analysis
Genomic DNA was prepared from microdissected tissue areas. Approximately 1000–5000 cells were microdissected from 5 µm histological sections of formalin-fixed, paraffin-embedded tissue. Normal tissue microdissected from the same specimen was used as control samples for each patient. The DNA extraction was performed using the QIAamp DNA FFPE tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. DNA was measured using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
TERT promoter mutation detection was carried out using a LightCycler 2.0 real-time polymerase chain reaction (PCR) system (Roche Diagnostics, Basel, Switzerland), as previously described [10,11,12]. Forward and reverse primers were designed to amplify DNA products encompassing C228T and C250T hotspot mutations in the TERT promoter 124 and 146 base pairs upstream of the ATG starting site, respectively. Real-time PCR was performed in 20 µl reaction mixtures containing 10 ng of genomic DNA, 0.1 µM forward and reverse primers, 0.2 µM of each nucleotide, and 1.5 units of OneTaq DNA polymerase in GC Buffer (New England Biolabs, Ipswich, MA, USA). The PCR included one cycle denaturation at 95 °C for 6 min and 45 cycles of denaturation at 94 °C for 20 s, annealing at 53 °C for 20 s, extension at 68 °C for 40 s. The primer and probe sequences were as following: TERT forward primer 5′-CGGGCTCCCAGTGGATTC-3′; TERT reverse primer 5′-CAGCGCTGCCTGAAACTC-3′; TERT C228T donor probe 5′-CGACCCCTCCCGGGTCCCCG-Flc-3′; TERT C228T acceptor probe 5′-LC640-AGCCCCTTCCGGGCCCTCCCAG-phos-3′; TERT C250T donor probe 5′-GCGCGGACCCCGCCCCGTC-Flc-3′; TERT C250T acceptor probe 5′-LC640-CCCCTTCCGGGTCCCCGGCCC-phos-3′.
Melting analyses were performed in the original capillary with one cycle of 95 °C for 1 min, 40 °C for 2 min, and then ramped up to 70 °C at 0.1 °C/second. Melting curves of the PCR products were monitored by plotting the changes in fluorescence emission at 640 nm that occurred by gradual heating of the PCR product–probe complex [10]. The melting curve was analyzed by LightCycler version 2.0 software. A single melting curve peak with a melting temperature within a ± 2.5 °C range of the predicted melting point was interpreted as representing a pure, single amplicon. If two peaks were present and both peaks fell within ± 2.5 °C of the predicted melting temperatures for mutant and wildtype, the amplicon was interpreted as containing a mutant cell population and a wildtype population. Serial dilution tests with known positive DNA demonstrated that a mutant cell population >5% was consistently detected.
Statistical analysis
Student t, Pearson Chi-square, and Fisher exact tests were used in the statistical analysis of clinicopathological parameters and TERT promoter mutation status. Statistical significance was defined as P < 0.05 and all P values were two-tailed.
Results
Patient demographics and pathological characteristics
TERT promoter mutation status was analyzed in 46 primary nonurachal adenocarcinoma specimens including 16 cystectomy and 30 transurethral resection samples. Table 1 summarizes the patient characteristics and clinical findings.
Of the 46 cases of primary adenocarcinoma analyzed, 26 patients were male and 20 were female (M:F ratio, 1.1:1), with a median age of 64 years (range, 39–92 years) (Table 1). At the time of diagnosis, there were 26 (56%) pT1, six (13%) pT2, 10 (22%) pT3, and four (9%) pT4 cases. All pT1 lesions were diagnosed from transurethral resection specimens. In all pT1 cases, muscular propria invasion was not present. Among the 46 primary adenocarcinomas of the urinary bladder, 17 (37%) were enteric, 11 (24%) were mucinous, and 18 (39%) were mixed adenocarcinoma variants (Table 1).
Of the 30 cases of urothelial carcinoma with glandular differentiation analyzed, 26 patients were male and four were female (M:F ratio, 5.2:1), with a median age of 73 years (range, 57–88 years). Most patients were over 60 years of age (80%). The cohort included 14 cystectomy and 16 transurethral resection cases. At the time of diagnosis, there were six (20%) pT1, 19 (63%) pT2, and five (17%) pT3 cases (Table 2).
Compared to primary adenocarcinoma of bladder, urothelial carcinoma with glandular differentiation had a high male-to-female ratio (5.2:1 vs 1.1:1; P = 0.0021) and higher median ages (73 vs 64 years; P = 0.0065), respectively.
TERT promoter mutation in the primary adenocarcinoma of urinary bladder
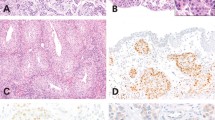
The prevalence of TERT promoter mutations C228T and C250T in primary adenocarcinoma of the urinary bladder was 33% (15/46 cases), among which 13 cases involved C228T (13/15, 87%) and 2 involved C250T (2/15, 13%) mutations. The incidence of TERT promoter mutations in histological variants of primary adenocarcinoma was 29% in enteric, 55% in mucinous, and 22% in mixed adenocarcinoma (Fig. 1; Table 1). The TERT mutation incidence among the histological variants was not statistically significant. There was no significant correlation of TERT promoter mutation with any clinicopathologic characteristics when considering all specimens or cystectomy specimens alone.
Hematoxylin and eosin-stained sections of enteric adenocarcinoma A, mucinous variant B, and mixed variant C show C228T TERT mutation D, E, F as indicated by a distinct melting temperature demonstrated by the red vertical bar. The TERT wildtype G, H, I corresponding to D, E, F, respectively, is demonstrated by the green vertical bar. The mutant and wildtype melting points have a 4 °C difference. In the cohort, 33% of the primary adenocarcinomas of bladder were positive for TERT promoter mutations.
We investigated KRAS mutations in a previous study [13]. TERT promoter mutations were absent in three KRAS-positive adenocarcinomas of the urinary bladder.
TERT promoter mutations in bladder urothelial carcinoma with glandular differentiation
TERT promoter mutations were detected in 20 (67%) of each paired urothelial carcinoma and glandular differentiation components, including 19 C228T and one C250T (Fig. 2). Identical TERT promoter mutation status was shared by the urothelial carcinoma and the glandular components in all cases, whether positive or negative for C228T or C250T mutations (Fig. 2).
Hematoxylin and eosin-stained sections from two urothelial carcinoma components A, C and coexisting glandular components B, D showed concordant TERT promoter mutations in either C228T E, F or C250T G, H as demonstrated by the distinct melting temperature indicated by the red vertical bar. The corresponding wildtype I, J, K, L is indicated by the green vertical bar. The mutant and wildtype melting points have a 2.5–4 °C difference. In the cohort, 67% of urothelial carcinomas with glandular differentiatin were positive for TERT promoter mutations. M Correlation between TERT promoter mutations in urothelial carcinoma and glandular components. The TERT mutation status in 30 urothelial carcinomas (upper row) with glandular components (lower row) was lined up and color coded. Each column represents a case. The TERT promoter mutation status was highly concordant whether mutated or not.
Compared to primary adenocarcinomas of bladder, urothelial carcinomas with glandular differentiation had a higher incidence of TERT mutation (33% vs 67%; P = 0.0048).
TERT promoter mutations in nephrogenic adenoma, villous adenoma, florid cystitis glandularis, and intestinal metaplasia of the bladder
Additionally, we analyzed TERT promoter mutations in nephrogenic adenoma (n = 24), villous adenoma (n = 8), florid cystitis glandularis (n = 31), and intestinal metaplasia (n = 20) of the bladder (n = 20). None of these lesions had TERT promoter mutations.
Discussion
Primary adenocarcinoma of the urinary bladder is a rare malignancy, accounting for 0.5–2.0% of all malignant vesical tumors found in patients in their sixth and seventh decades of life, and characterized by an aggressive behavior [1, 2]. One-third of the primary adenocarcinomas of the urinary bladder have advanced cancer with or without lymph node metastasis at the time of diagnosis [14, 15]. These cancers also occur in association with schistosomiasis, endometriosis, bladder augmentation, and other irrigative conditions of the urinary bladder. Primary bladder adenocarcinoma has a variety of growth patterns, pure or mixed, including enteric, mucinous, and mixed patterns [3]. The morphological features of these cancers are identical to those from other organs. Urothelial carcinomas also have diverse morphological manifestations, including glandular differentiation [16]. Although studies have identified common molecular pathways in conventional urothelial carcinoma, molecular data are lacking for adenocarcinomas of the urinary bladder [7, 8, 17, 18].
Mutations in the TERT promoter have recently been implicated in urothelial carcinogenesis and are present in 60–80% of conventional and variants of urothelial carcinomas [4,5,6, 9, 19]. TERT promoter mutations are proposed as the earliest genetic alterations in bladder carcinogenesis [20]. TERT promoter mutations contribute to tumorigenesis by promoting immortalization and genomic instability in two phases [21]. The TERT promoter mutation analysis may be a useful adjunct test in difficult cases [4, 7, 9, 10, 12, 22,23,24,25,26,27,28,29,30,31,32,33]. To investigate the degree to which TERT promoter mutations are involved in adenocarcinoma of the bladder, and to gain insight into the potential of linked tumorigenesis to conventional urothelial carcinoma, we evaluated TERT promoter mutations in a series of 46 cases of primary nonurachal adenocarcinoma of urinary bladder and compared to a cohort containing 30 urothelial carcinomas with glandular differentiation. We found TERT promoter mutations in 33% (15/46) of primary adenocarcinomas of the urinary bladder, providing evidence that some adenocarcinoma of the urinary bladder followed a common tumorigenesis pathway. However, the incidence of TERT mutations was far less than those observed in urothelial carcinoma with glandular differentiation (33% vs 67% in current study).
There were conflicting data on incidence of TERT promoter mutation in primary adenocarcinoma of the urinary bladder. In a study by Vail and colleagues, TERT promoter mutation was absent in an analysis of 10 cases of primary adenocarcinoma [32]. TERT promoter mutation is also rare (4%) in urachal adenocarcinoma [34]. In contrast, Cowan et al. investigated 14 cases of primary adenocarcinomas of urinary bladder and found 29% had TERT mutations [29]. These discrepancies may reflect differences in patient population, tumor location (urachal vs nonurachal), sample size, methodology divergence, tumorigenesis, and biology. Together, these data suggested that TERT promoter mutation may not be the only driver activation event in the carcinogenesis of primary adenocarcinoma. We also found TERT promoter mutations from 67% of urothelial carcinomas with glandular differentiation, in which the concordant TERT promoter mutation status was seen in all cases (Fig. 2). Our findings support that both glandular and urothelial carcinoma components were derived from a common clonal origin.
Glandular lesions of the urinary bladder are frequently encountered. The major benign glandular lesions in the differential diagnosis include nephrogenic adenoma, villous adenoma, florid cystitis glandularis, and intestinal metaplasia of the bladder [1, 2, 35]. None of these benign glandular lesions harbored TERT promoter mutations in the current study. The distinction of benign glandular lesions from malignant tumors is usually straightforward, but it could be diagnostically challenging in some cases. Likewise, some malignant urothelial tumors may also mimic adenocarcinoma of the bladder. One example is nested variant of urothelial carcinoma. High incidence of TERT promoter mutation was found in nested variant urothelial carcinoma in recent studies [9, 31, 33]. These data suggest that TERT promoter mutation analysis could potentially be helpful in the differential diagnosis of glandular lesions of the urinary bladder (Fig. 3).
TERT promoter mutations exhibited high organ specificity. Metastatic adenocarcinoma of bladder from cancers originating from prostate, colorectal, breast, and lung rarely harbor TERT mutations [11, 32, 34, 36,37,38,39]. Wu et al. screened 302 patients with a variety of urogenital cancers and determined the clinical relevance of TERT promoter mutations in urogenital cancer [40]. Urothelial carcinomas showed the highest mutation incidence, but nonurothelial cancers showed very low or no mutation [40]. TERT genotypes have been shown to be conserved across spatially, temporally, and morphologically distinct components of a single urothelial tumor, further supporting its use as a relatively stable and reliable molecular biomarker [41]. Urachal adenocarcinoma can have a variety of morphological appearances and can secondarily involve the bladder. Thiem et al. investigated 23 urachal adenocarcinomas and found TERT promoter mutation only in one case (4%) [34]. In contrast, a high percentage of primary bladder adenocarcinomas harbor TERT promoter mutation. These findings further support the utility of TERT promoter mutation test as a differential diagnosis tool.
In summary, TERT promoter mutations were observed in 33% of primary adenocarcinomas of the urinary bladder and in 67% of urothelial carcinomas with glandular differentiation. Identical TERT promoter mutation status in both urothelial and glandular components from the same tumor indicate a common clonal origin. TERT promoter mutation analysis may be a useful ancillary study in the differential diagnosis.
Data availability
Original experimental data are available upon request.
References
Cheng L, Lopez-Beltran A, Bostwick DG. Bladder Pathology. Hoboken, NJ: Wiley-Blackwell; 2012.
Williamson SR, Lopez-Beltran A, Montironi R, Cheng L. Glandular lesions of the urinary bladder: clinical significance and differential diagnosis. Histopathology. 2011;58:811–34.
Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO classification of tumours of the urinary system and male genital organ. (International Agency for Research on Cancer (IARC): Lyon, France, IARC Press, 2016.
Bell RJ, Rube HT, Xavier-Magalhaes A, Costa BM, Mancini A, Song JS, et al. Understanding TERT promoter mutations: a common path to immortality. Mol Cancer Res. 2016;14:315–23.
Allory Y, Beukers W, Sagrera A, Flandez M, Marques M, Marquez M, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65:360–6.
Gunes C, Wezel F, Southgate J, Bolenz C. Implications of TERT promoter mutations and telomerase activity in urothelial carcinogenesis. Nat Rev Urol. 2018;15:386–93.
Warrick JI, Knowles MA, Yves A, van der Kwast T, Grignon DJ, Kristiansen G, et al. Report from the International Society of Urological Pathology (ISUP) Consultation Conference on molecular pathology of urogenital cancers. II. Molecular pathology of bladder cancer: progress and challenges. Am J Surg Pathol. 2020;44:e30–46.
Kamoun A, de Reynies A, Allory Y, Sjodahl G, Robertson AG, Seiler R, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol. 2020;77:420–33.
Levy DR, Cheng L. The expanding molecular and mutational landscape of nested variant of urothelial carcinoma. Histopathology. 2020;76:638–9.
Cheng L, Davidson DD, Wang M, Lopez-Beltran A, Montironi R, Wang L, et al. Telomerase reverse transcriptase (TERT) promoter mutation analysis of benign, malignant and reactive urothelial lesions reveals a subpopulation of inverted papilloma with immortalizing genetic change. Histopathology. 2016;69:107–13.
Wang X, Lopez-Beltran A, Osunkoya AO, Wang M, Zhang S, Davidson DD, et al. TERT promoter mutation status in sarcomatoid urothelial carcinomas of the upper urinary tract. Future Oncol. 2017;13:705–14.
Cheng L, Montironi R, Lopez-Beltran A. TERT promoter mutations occur frequently in urothelial papilloma and papillary urothelial neoplasm of low malignant potential. Eur Urol. 2017;71:497–8.
Alexander RE, Lopez-Beltran A, Montironi R, MacLennan GT, Post KM, Bilbo SA, et al. KRAS mutation is present in a small subset of primary urinary bladder adenocarcinomas. Histopathology. 2012;61:1036–42.
Rogers CG, Palapattu GS, Shariat SF, Karakiewicz PI, Bastian PJ, Lotan Y, et al. Clinical outcomes following radical cystectomy for primary nontransitional cell carcinoma of the bladder compared to transitional cell carcinoma of the bladder. J Urol. 2006;175:2048–53.
Zaghloul MS, Nouh A, Nazmy M, Ramzy S, Zaghloul AS, Sedira MA, et al. Long-term results of primary adenocarcinoma of the urinary bladder: a report on 192 patients. Urol Oncol. 2006;24:13–20.
Lopez-Beltran A, Cheng L. Histologic variants of urothelial carcinoma: differential diagnosis and clinical implications. Hum Pathol. 2006;37:1371–88.
Cheng L, Eble JN. Molecular Surgical Pathology. New York, NY: Springer; 2013.
Cheng L, Zhang S, MacLennan GT, Williamson SR, Lopez-Beltran A, Montironi R. Bladder cancer: translating molecular genetic insights into clinical practice. Hum Pathol. 2011;42:455–81.
Hurst CD, Platt FM, Knowles MA. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol. 2014;65:367–9.
Kinde I, Munari E, Faraj SF, Hruban RH, Schoenberg M, Bivalacqua T, et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013;73:7162–7.
Chiba K, Lorbeer FK, Shain AH, McSwiggen DT, Schruf E, Oh A, et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science. 2017;357:1416–20.
Kurtis B, Zhuge J, Ojaimi C, Ye F, Cai D, Zhang D, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol. 2016;21:7–11.
Zhong M, Tian W, Zhuge J, Zheng X, Huang T, Cai D, et al. Distinguishing nested variants of urothelial carcinoma from benign mimickers by TERT promoter mutation. Am J Surg Pathol. 2015;39:127–31.
Maurer A, Ortiz-Bruechle N, Guricova K, Rose M, Morsch R, Garczyk S, et al. Comparative genomic profiling of glandular bladder tumours. Virchows Arch. 2020;477:445–54.
Taylor AS, McKenney JK, Osunkoya AO, Chan MP, Al-Ahmadie HA, Spratt DE, et al. PAX8 expression and TERT promoter mutations in the nested variant of urothelial carcinoma: a clinicopathologic study with immunohistochemical and molecular correlates. Mod Pathol. 2020;33:1165–71.
Acosta AM, Sholl LM, Fanelli GN, Gordetsky JB, Baniak N, Barletta JA, et al. Intestinal metaplasia of the urinary tract harbors potentially oncogenic genetic variants. Mod Pathol. 2020 https://doi.org/10.1038/s41379-020-00655-z
Akgul M, MacLennan GT, Cheng L. Distinct mutational landscape of inverted urothelial papilloma. J Pathol. 2019;249:3–5.
Cowan M, Springer S, Nguyen D, Taheri D, Guner G, Rodriguez MA, et al. High prevalence of TERT promoter mutations in primary squamous cell carcinoma of the urinary bladder. Mod Pathol. 2016;29:511–5.
Cowan ML, Springer S, Nguyen D, Taheri D, Guner G, Mendoza Rodriguez MA, et al. Detection of TERT promoter mutations in primary adenocarcinoma of the urinary bladder. Hum Pathol. 2016;53:8–13.
Isharwal S, Hu W, Sarungbam J, Chen YB, Gopalan A, Fine SW, et al. Genomic landscape of inverted urothelial papilloma and urothelial papilloma of the bladder. J Pathol. 2019;248:260–5.
Taylor AS, McKenney JK, Osunkoya AO, Chan MP, Al-Ahmadie HA, Spratt DE, et al. PAX8 expression and TERT promoter mutations in the nested variant of urothelial carcinoma: a clinicopathologic study with immunohistochemical and molecular correlates. Mod Pathol. 2020;33:1165–71.
Vail E, Zheng X, Zhou M, Yang X, Fallon JT, Epstein JI, et al. Telomerase reverse transcriptase promoter mutations in glandular lesions of the urinary bladder. Ann Diagn Pathol. 2015;19:301–5.
Weyerer V, Weisser R, Moskalev EA, Haller F, Stoehr R, Eckstein M, et al. Distinct genetic alterations and luminal molecular subtype in nested variant of urothelial carcinoma. Histopathology. 2019;75:865–75.
Thiem S, Herold T, Krafft U, Bremmer F, Tolkach Y, Szasz AM, et al. Telomerase reverse transcriptase (TERT) promoter mutations are rare in urachal cancer. Pathol Int. 2017;67:597–601.
Cheng L, MacLennan GT, Bostwick DG. Urologic Surgical Pathology. 4th ed. Philadelphia, PA: Elsevier; 2020.
Stoehr R, Taubert H, Zinnall U, Giedl J, Gaisa NT, Burger M, et al. Frequency of TERT promoter mutations in prostate cancer. Pathobiology. 2015;82:53–57.
Cruvinel-Carloni A, Yamane L, Scapulatempo-Neto C, Guimaraes D, Reis RM. Absence of TERT promoter mutations in colorectal precursor lesions and cancer. Genet Mol Biol. 2018;41:82–84.
Priemer DS, Wang M, Zhang S, Lopez-Beltran A, Kouba E, Montironi R. et al.Small-cell carcinomas of the urinary bladder and prostate: TERT promoter mutation status differentiates sites of malignancy and provides evidence of common clonality between small-cell carcinoma of the urinary bladder and urothelial carcinoma.Eur Urol Focus. 2018;4:880–8.
Wang K, Liu T, Liu L, Liu J, Liu C, Wang C, et al. TERT promoter mutations in renal cell carcinomas and upper tract urothelial carcinomas. Oncotarget. 2014;5:1829–36.
Wu S, Huang P, Li C, Huang Y, Li X, Wang Y, et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: a genomic and molecular study. Eur Urol. 2014;65:274–7.
Brown NA, Lew M, Weigelin HC, Weizer AZ, Montgomery JS, Betz BL, et al. Comparative study of TERT promoter mutation status within spatially, temporally and morphologically distinct components of urothelial carcinoma. Histopathology. 2018;72:354–6.
Author information
Authors and Affiliations
Contributions
LC, AL-B, and SZ are involved in conception and design of the paper. MW, SZ, and LC are responsible for data acquisition, data analysis, and writing the article. All the authors (LC, AL-B, MW, RM, HZK, and SZ) read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics statement
This research was approved by the Institutional Review Boards in accordance with the Institutional Committee for the Protection of Human Subjects.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, L., Lopez-Beltran, A., Wang, M. et al. Telomerase reverse transcriptase (TERT) promoter mutations in primary adenocarcinoma of bladder and urothelial carcinoma with glandular differentiation: pathogenesis and diagnostic implications. Mod Pathol 34, 1384–1391 (2021). https://doi.org/10.1038/s41379-021-00776-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00776-z