Abstract
Acral lentiginous melanoma (ALM) is a rare type of cutaneous melanoma with a poor prognosis. It is unclear whether the poor outcome of ALM is due to its inherent disease characteristics or advanced stage at initial diagnosis. To address this question, we retrospectively analyzed the clinicopathologic factors of 828 thin (T1; Breslow thickness ≤1.0 mm) melanomas [129 (15.6%) ALMs and 699 (84.4%) non-ALMs] and their nodal and distance metastases and local recurrence rates and determined their relationship with the disease-specific (DSS), overall (OS), and recurrence-free survivals (RFS) at the pathologic stages T1, T1a, and T1b with a median follow-up time of 84.5 months. With the exception of OS at T1b stage, ALM patients showed significantly lower 5- and 10-year DSS, OS, and RFS rates at every pathologic stage when compared with non-ALM. In multivariable analysis, ALM histologic type, SLN positivity, age, and the use of systemic therapy were detected as independent poor prognostic factors associated with significantly lower survival rates. ALM histologic type was associated with lower DSS and OS rates at T1 and T1a stages and lower RFS rates at T1b stage. SLN positivity was associated with lower DSS, OS, and RFS rates at T1, T1a, and T1b stages. Age was associated with lower OS rates at T1 and T1b stages. Whereas the use of systemic therapy was associated with lower DSS rates at T1a stage and RFS rates at T1b stage. In addition, the ALM group showed significantly older median age patients and higher rates of female sex, Hispanic ethnicity, nevoid cytology, non-brisk tumor-infiltrating lymphocytes, nodal metastasis, and local recurrence at every pathologic stage of thin melanoma. Our findings suggest that ALM is inherently more aggressive than other types of cutaneous melanoma. This information may be useful for prognostic stratification of patients with thin melanomas, especially to help guide the clinical decision-making for SLN biopsy and patients entering clinical trials.
Similar content being viewed by others
Introduction
Acral lentiginous melanoma (ALM) is a rare type of cutaneous melanoma that arises on the acral skin of the palms, soles, and digits as well as the nail unit and has a characteristic histologic pattern of confluent proliferation of predominantly single-cell units of neoplastic melanocytes [1, 2]. Compared with other histologic types of cutaneous melanoma, ALM is associated with delayed diagnosis [3], advanced Breslow thickness, a higher rate of ulceration, greater propensity for lymph node and distant metastases, and ultimately worse prognosis [4, 5].
Alternatively, thin melanoma is defined as cutaneous melanoma of any histologic type with Breslow thickness up to 1.0 mm and staged as T1 in the tumor-nodes-metastases staging system [6]. Thin melanoma is known to have up to 95% 10-year survival rates [7, 8] and low rates of nodal and distant metastases estimated as <5% for T1a melanomas and 5–12% for T1b melanomas [9,10,11,12].
Due to the uncertain benefit and low rates of nodal metastasis, SLN biopsy is not routinely performed for patients with thin melanoma [13], but the National Comprehensive Cancer Network recommends “discussion and consideration” of SLN biopsy for patients with T1b melanoma, particularly when it is associated with high-risk features such as ulceration, dermal mitosis, or regression [14, 15]. Other prognostic factors can also be taken into account in patient selection for SLN biopsy, including the presence of lymphovascular invasion and satellitosis [16, 17]. A recent study of thin melanoma showed that ALM histologic type was associated with a significantly increased risk of SLN metastasis [18]. However, the prognostic significance of ALM histologic type on patient’s survival in thin melanoma is not well explored.
To clarify whether the worse outcome of ALM is due to its inherent disease characteristics or higher stage at initial diagnosis, we investigated clinicopathologic factors in a large series of thin ALMs and non-ALMs. We included only thin melanomas (i) to limit the powerful prognostic impact of Breslow thickness and its confounding effect on other prognostic factors, such as ulceration, mitosis, and nodal metastasis, (ii) to limit the impact of delayed diagnosis, (iii) to limit the prognostic impact of nodal metastasis and ulceration, which are known to be at low prevalence rates in thin melanoma, and ultimately (iv) to provide helpful prognostic information that might be used for risk stratification, especially to help guide the clinical decision-making for SLN biopsy and for patients entering clinical trials.
Materials and methods
Case selection
With approval from our Institutional Review Board, we searched the archives of the Department of Pathology, The University of Texas MD Anderson Cancer Center, for records containing the terms “acral”, “lentiginous”, and “melanoma” (all three terms) but not containing “metastatic” to identify cases of primary ALM and for records containing the terms “cutaneous” and “melanoma” but not containing either “lentiginous” or “metastatic” to identify cases of primary cutaneous melanoma of other histologic types. The search was limited to melanomas that were diagnosed, treated, and followed up at our institution during 1994–2009. From the search results, T1 melanomas were selected and microstaged into T1a and T1b groups according to the eighth edition of the American Join Committee on Cancer staging system for cutaneous melanoma [6].
Collection of clinical and histologic data
The original diagnosis of melanoma was made by at least 1 and up to all 7 of the participating dermatopathologists (PPA, JLC, DI, PN, VGP, MTT, and CAT-C), and the retrieved material was re-evaluated by PPA. For each patient, the following details were recorded: date of initial diagnosis, sex, age at diagnosis, race/ethnicity, anatomic site of the primary lesion, date of last follow-up, cause of death, histologic type, Breslow thickness, Clark level, mitotic rate, ulceration, regression, lymphovascular invasion, perineural invasion, tumor-infiltrating lymphocytes, microsatellitosis, vertical growth phase, predominant cytology, the status of SLNs and non-SLNs, the status of resection margins, local recurrence, and distant metastasis. Regression was defined histologically as the presence of an area of reduced number of dermal melanoma cells in association with a variably dense lymphohistiocytic infiltrate, melanophages, increased number of capillaries, and superficial dermal fibrosis, and overlined by irregularly attenuated epidermis. The status of regression was recorded as either present or absent irrespective of its stage or extent.
Lymphovascular invasion was predominantly detected by routine hematoxylin and eosin (H&E) staining method. However, for equivocal cases with suspicious lymphovascular invasion, an additional immunohistochemical study with a vascular marker such as CD31 or D2–40 was performed for the definitive interpretation. The status of regional lymph node metastasis was determined using our institutional protocol of lymph node examination for cutaneous melanoma. The protocol required an initial evaluation with H&E-stained sections only. If no obvious nodal metastasis was identified, three sections were subsequently cut at ~200 µm into the block and placed on slides. One slide was stained with H&E, one slide was stained with panmelanocytic cocktail (HMB45, tyrosinase, and MART1), and one slide was reserved as an unstained slide for any further ancillary study that might be needed.
Due to the rarity of its use in thin melanoma, systemic therapy was defined by the use of chemotherapy, radiotherapy, and immunotherapy, each alone or in combination. The status of the use of systemic therapy was reported as either present or absent.
Statistical methods
Clinicopathologic characteristics were classified by the T group (T1, T1a, and T1b). Categorical variables were summarized by frequencies and percentages and compared between groups using either the Fisher’s exact test or its generalization. Continuous variables were summarized by medians and ranges (minimums, maximums) and compared between groups using the Wilcoxon rank-sum test.
Median follow-up time from the time of diagnosis was 84.5 months. DSS and OS were computed from the date of diagnosis to the date of death or the last follow-up. For DSS, the death date for patients who died from causes other than melanoma and those alive at their last follow-up date were administratively censored. For OS, patients alive at their last follow-up date were administratively censored. RFS was computed from the date of diagnosis to the date of local recurrence or date of death from any cause. Patients alive at their last follow-up date who did not experience local recurrence were administratively censored. The Kaplan–Meier method was used to estimate DSS, OS, and RFS, and the log-rank test was used to assess differences between groups. Univariate Cox proportional hazards regression models were used to assess the association between clinicopathologic factors and survival. A multivariable Cox proportional hazards regression model was determined using backward elimination with an exit criterion of p ≥ 0.05. All statistical analyses were performed using SAS 9.4 for Windows (SAS Institute Inc., Cary, NC) and used a significance level of 5%.
Results
Sampling and categorization
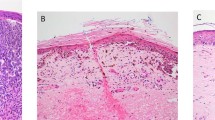
A total of 828 T1 melanomas were retrieved: 129 (15.5%) ALMs (Fig. 1a) and 699 (84.5%) non-ALMs (superficial spreading, lentigo maligna, and nodular melanomas) (Fig. 1b–d). Of the 129 T1 ALMs, 82 (63.5%) were T1a and 47 (36.5%) were T1b melanomas. Of the 699 T1 non-ALMs, 454 (65%) were T1a and 245 (35%) were T1b melanomas.
Histologic features of four morphologic subtypes of cutaneous melanoma (a) acral lentiginous type with Breslow thickness 1.00 mm, pT1b (×40 magnification) (b) superficial spreading type with Breslow thickness 0.65 mm, pT1a (×40 magnification) (c) lentigo maligna type with Breslow thickness 0.86 mm, pT1b (×100 magnification) and (d) nodular type with Breslow thickness 0.71 mm, pT1a (×100 magnification).
Clinicopathologic characteristics
The clinicopathologic characteristics of thin melanomas were categorized into ALM and non-ALM groups, classified as T1, T1a, and T1b, and summarized in Table 1. The ALM group (n = 129) is purely comprised of ALMs (n = 129, 100%), whereas the non-ALM group (n = 699) is comprised of 617 (88%) superficial spreading melanomas, 60 (9%) lentigo maligna melanomas, and 22 (3%) nodular melanomas. Of the 7 (15%) T1b ALM with ulceration, 3 (6%) were <0.8 mm Breslow thickness and of the 21 (9%) T1b non-ALM with ulceration, 10 (45%) were <0.8 mm Breslow thickness.
At all pathologic stages, ALMs patients were associated with significantly older median age and higher rates of female sex, Hispanic ethnicity, nevoid cytology, non-brisk tumor-infiltrating lymphocytes, nodal (SLN and non-SLN) metastasis, and local recurrence in comparison to non-ALM patients. Alternatively, non-ALMs patients were associated with significantly higher rates of Caucasian ethnicity, Clark level III, presence of vertical growth phase, epithelioid cytology, and regression.
Survival rates
DSS rates
At all pathologic stages, ALM patients were associated with significantly lower 5- and 10- year DSS rates than non-ALM patients. The 5- and 10-year DSS rates for ALMs were 90% and 82%, respectively, for T1, 92% and 79%, respectively, for T1a, and 86% and 86%, respectively, for T1b. Whereas the 5- and 10-year DSS rates for non-ALMs were 99% and 98%, respectively, for T1, 100% and 99%, respectively, for T1a, and 99% and 95%, respectively, for T1b (Fig. 2a–c).
Survival analyses of patients with thin melanomas stratified by the histologic type (ALM vs. non-ALM) and demonstrated by Kaplan–Meier curves for DSS (a–c), OS (d–f), and RFS (g–i) at the pathologic stages T1, T1a, and T1b. With exception to the OS of T1b melanomas, ALM patients were associated with significantly lower survival rates for DSS, OS, and RFS at the T1, T1a, and T1b pathologic stages.
Factors associated with DSS
By univariate analysis, ALM histologic type, SLN metastases, and the use of systemic therapy were associated with a significantly higher risk of melanoma-specific death in T1, T1a, and T1b melanomas, whereas Breslow thickness and lack of regression were associated with a significantly higher risk of melanoma-specific death in T1 ALM (Table 2).
By multivariable analysis, independent poor prognostic factors associated with significantly increased risk of melanoma-specific death included the ALM histologic type in T1 and T1a melanomas, the use of systemic therapy for T1b melanomas, and SLN metastasis in T1, T1a, and T1b melanomas (Table 2).
OS rates
At T1 and T1a stages, ALM patients were associated with significantly lower 5- and 10-year OS rates than non-ALM patients. The 5- and 10-year OS rates for ALMs were 81% and 67%, respectively, for T1 and 81% and 65%, respectively, for T1a. Whereas the 5- and 10-year OS rates for non-ALMs were 96% and 87%, respectively, for T1, and 96% and 91%, respectively, for T1a. At the T1b stage, no significant difference in the OS between the ALM and non-ALM groups was identified and the 5- and 10- year OS rates were 81% and 69%, respectively, for ALM and 94% and 80%, respectively for non-ALM (Fig. 2d–f).
Factors associated with OS
By univariate analysis, factors associated with a significantly higher risk of death were ALM histologic type for T1 and T1a melanomas, Breslow thickness and male sex in T1 melanoma, Hispanic ethnicity in T1a melanoma, and age and SLN metastasis as well as the use of systemic therapy in T1, T1a, and T1b melanomas (Table 3). By multivariable analysis, independent poor prognostic factors associated with significantly increased risk of death included age in T1 and T1a melanomas, and SLN metastasis in T1, T1a, and T1b melanomas (Table 3).
RFS rates
At all pathologic stages, ALM patients were associated with significantly lower 5- and 10-year RFS rates than non-ALM patients. The 5- and 10-year RFS rates for ALMs were 77% and 62%, respectively, for T1, 81% and 65%, respectively, for T1a, and 72% and 57%, respectively, for T1b. Whereas the 5- and 10-year RFS rates for non-ALMs were 95% and 87%, respectively, for T1, 96% and 90%, respectively, for T1a, and 94% and 80%, respectively, for T1b (Fig. 2g–i).
Factors associated with RFS
By univariate analysis, ALM type, age, SLN metastasis, number of positive SLNs, and the use of systemic therapy were associated with a significantly higher risk of melanoma-recurrence rate in T1, T1a, and T1b melanomas, whereas Breslow thickness and nevoid cytomorphology were associated with a significantly higher risk of melanoma-recurrence rate in T1 melanomas, and Hispanic ethnicity was associated with a significantly higher risk of melanoma-recurrence rate in T1a melanomas (Table 4).
By multivariable analysis, independent poor prognostic factors associated with significantly increased risk of melanoma-recurrence rate included ALM histologic type in T1b melanomas, age for T1 and T1b melanomas, and SLN metastases in T1, T1a, and T1b melanomas (Table 4).
Discussion
In this current study of thin melanoma, with the only exception of the OS rates for T1b melanomas, ALM patients were associated with significantly lower 5- and 10-year DSS, OS, and RFS rates than non-ALM patients. The ALM histologic type, SLN positivity, patient’s age, and the use of systemic therapy were detected as independent prognostic factors associated with poor prognosis.
In T1 melanomas, we found the histologic type of ALM as an independent prognostic factor for lower rates of DSS. When we microstaged the T1 melanomas, we found that ALM type was both dependent and independent predictor of worse DSS in T1a but only as a dependent poor prognostic factor in T1b melanomas. This inconsistency in the prognostic impact of ALM histologic type across pathologic stages of thin melanoma might be explained by the relatively powerful prognostic impact of greater Breslow thickness and/or the presence of ulceration confounding the prognostic impact of ALM histologic type in T1b melanomas. For RFS, the ALM histologic type was detected as a dependent prognostic factor for lower rates at the T1, T1a, and T1b pathologic stages, and as an independent prognostic factor for T1b melanomas.
Alternatively, SLN positivity was consistently detected as both dependent and independent prognostic factor for worse DSS, OS, and RFS in every pathologic stage of thin melanomas in concordance with the previously reported studies of ALM [5, 19,20,21,22].
In comparison to our current study, Marek et al. [18]. performed a comparable yet less extensive analysis of thin melanomas to detect clinicopathologic factors that can predict SLN metastasis. The authors reported ALM histologic type, mitosis, and Clark level IV–V of invasion as independent predictors of increased SLN metastasis in thin melanoma. However, the study was limited by the small sample size of ten thin ALMs and the absence of analysis of the study findings’ prognostic impact on patient’s survival.
Although Teramoto et al. [19]. reported that older patient age as an independent poor prognostic factor for DSS, most previous single-institution studies of ALM revealed no prognostic impact of age on either DSS or OS [23,24,25]. In our current study, we found that older age at the time of diagnosis as an independent prognostic factor for OS and RFS in T1 and T1a melanomas. However, this impact of age on OS and RFS might be confounded by other unexamined prognostic factors in our patient population, such as patients’ general health, socioeconomic status, and access to healthcare.
Similar to Häfliger et al. [10]. who reported poor response to systemic and targeted therapy in advanced stage ALMs that ultimately associated with lower OS, in our current study, the use of systemic therapy was associated with worse DSS of T1a melanomas only. Although the latter result might be explained by the patients’ advanced disease stage, it is limited by the small number of patients who received systemic therapy.
In agreement with previously reported studies of ALM, the ALM patients of our current study were significantly older than non-ALM patients [26,27,28] and had significantly higher rates of female sex [29], Hispanic ethnicity [4], non-brisk tumor-infiltrating lymphocytes [30], nodal metastasis [10, 18, 21], and local recurrence presented across all tumor stages of thin melanoma [10]. Unique to our current study, significantly higher rates of nevoid cytomorphology were detected in the ALM group than in the non-ALM group.
The findings of our current study indicating ALM as an inherently aggressive type of cutaneous melanoma might be supported by the unique histologic characteristic of the acral skin as well as explained by the molecular signature of ALM unrelated to sun exposure [31]. Histologically, the epidermis of acral melanoma is usually thicker than that of non-acral melanomas. Hence, when only a small volume of tumor is present within the dermis, an acral melanoma may have a greater tumor thickness than when a similar volume of tumor is present in the dermis in a melanoma occurring at a non-acral site. At the molecular level, ALMs have less frequent mutations of BRAF and NRAS compared with other types of cutaneous melanoma [32]. Alternatively, activating mutations in KIT and PDGFRA as well as several genetic changes such as copy number gains in CCNDI and TERT were detected at higher rates in ALM [31, 33,34,35]. A recent study found amplification of TRET detected by FISH analysis is increased in metastatic ALM, suggesting a role in disease progression [36].
The strengths of our current study include the large sample size of thin melanoma as well as the comprehensive assessment of multiple clinicopathologic factors and the determination of their impact on DSS, OS, and RFS. The limitations of our current study include the fact that it was performed at a single referral cancer center, which introduces the possibility of referral bias. In addition, due to the rarity of non-ALMs arising on the acral skin such as nodular melanomas, these types of acral melanomas were not investigated in our current study of thin melanoma.
The significantly lower rates of DSS, OS, and RFS and a higher rate of SLN metastasis detected in thin ALM argue against the concept that thin melanoma is associated with an excellent prognosis and low rates of nodal metastases irrespective of its histologic type. The identification of ALM type as an independent poor prognostic factor in thin melanoma indicates that ALM is an inherently aggressive disease.
Together with other prognostic factors, ALM histologic type may provide helpful prognostic information for risk stratification, such as for patients with thin melanoma being considered for SLN biopsy and patients entering clinical trials. Nonetheless, due to our study limitations, further studies, especially at the molecular level, are warranted to validate our findings.
References
Reed RJ. Acral lentiginous melanoma. In: Reed RJ, editor. New concepts in surgical pathology of the skin. New York: John Wiley and Sons; 1976. p. 89–90.
Desai A, Ugorji R, Khachemoune A. Acral melanoma foot lesions. Part 1: epidemiology, aetiology, and molecular pathology. Clin Exp Dermatol. 2017;42:845–8.
Franke W, Neumann NJ, Ruzicka T, Schulte KW. Plantar malignant melanoma - a challenge for early recognition. Melanoma Res. 2000;10:571–6.
Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427–34.
Phan A, Touzet S, Dalle S, Ronger-Savlé S, Balme B, Thomas L. Acral lentiginous melanoma: a clinicoprognostic study of 126 cases. Br J Dermatol. 2006;155:561–9.
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma of the Skin. In: Amin AB, Edge SB, Greene FL, et al., editors. AJCC cancer staging manual. 8th Edn. New York: Springer; 2017. p. 563–85.
Gimotty PA, Elder DE, Fraker DL, Botbyl J, Sellers K, Elenitsas R, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol. 2007;25:1129–34.
Roncati L, Pusiol T, Piscioli F. Thin melanoma: a generic term including four histological subtypes of cutaneous melanoma. Acta Dermatovenerol Croat. 2016;24:169–74.
Han D, Zager JS, Shyr Y, Chen H, Berry LD, Iyengar S, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol. 2013;31:4387–93.
Häfliger EM, Ramelyte E, Mangana J, Kunz M, Kazakov DV, Dummer R, et al. Metastatic acral lentiginous melanoma in a tertiary referral center in Switzerland: a systematic analysis. Melanoma Res. 2018;28:442–50.
Andtbacka RH, Gershenwald JE. Role of sentinel lymph node biopsy in patients with thin melanoma. J Natl Compr Canc Netw. 2009;7:308–17.
Cordeiro E, Gervais MK, Shah PS, Look Hong NJ, Wright FC. Sentinel lymph node biopsy in thin cutaneous melanoma: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23:4178–88.
Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609.
Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE III, et al. Melanoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:450–73.
Rosko AJ, Vankoevering KK, McLean SA, Johnson TM, Moyer JS. Contemporary management of early-stage melanoma: a systematic review. JAMA Facial Plast Surg. 2017;19:232–8.
Petersson F, Diwan AH, Ivan D, Gershenwald JE, Johnson MM, Harrell R, et al. Immunohistochemical detection of lymphovascular invasion with D2-40 in melanoma correlates with sentinel lymph node status, metastasis and survival. J Cutan Pathol. 2009;36:1157–63.
Niakosari F, Kahn HJ, McCready D, Ghazarian D, Rotstein LE, Marks A, et al. Lymphatic invasion identified by monoclonal antibody D2-40, younger age, and ulceration: predictors of sentinel lymph node involvement in primary cutaneous melanoma. Arch Dermatol. 2008;144:462–7.
Marek AJ, Ming ME, Bartlett EK, Karakousis GC, Chu EY. Acral lentiginous histologic subtype and sentinel lymph node positivity in thin melanoma. JAMA Dermatol. 2016;152:836–7.
Teramoto Y, Keim U, Gesierich A, Schuler G, Fiedler E, Tüting T, et al. Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. Br J Dermatol. 2018;178:443–51.
Hieken TJ, Grotz TE, Comfere NI, Inselman JW, Habermann EB. The effect of the AJCC 7th edition change in T1 melanoma substaging on national utilization and outcomes of sentinel lymph node biopsy for thin melanoma. Melanoma Res. 2015;25:157–63.
Ito T, Wada M, Nagae K, Nakano-Nakamura M, Nakahara T, Hagihara A, et al. Acral lentiginous melanoma: who benefits from sentinel lymph node biopsy? J Am Acad Dermatol. 2015;72:71–7.
Egger ME, McMasters KM, Callender GG, Quillo AR, Martin RC II, Stromberg AJ, et al. Unique prognostic factors in acral lentiginous melanoma. Am J Surg. 2012;204:874–9. discussion 9–80.
Bello DM, Chou JF, Panageas KS, Brady MS, Coit DG, Carvajal RD, et al. Prognosis of acral melanoma: a series of 281 patients. Ann Surg Oncol. 2013;20:3618–25.
Kuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in caucasians: clinical features, histopathology and prognosis in 112 patients. Br J Dermatol. 2000;143:275–80.
Boriani F, O’Leary F, Tohill M, Orlando A. Acral Lentiginous Melanoma—misdiagnosis, referral delay and 5 years specific survival according to site. Eur Rev Med Pharm Sci. 2014;18:1990–6.
Ishihara K, Saida T, Otsuka F, Yamazaki N. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008;13:33–41.
Paolino G, Bekkenk MW, Didona D, Eibenschutz L, Richetta AG, Cantisani C, et al. Is the prognosis and course of acral melanoma related to site-specific clinicopathological features? Eur Rev Med Pharm Sci. 2016;20:842–8.
Lv J, Dai B, Kong Y, Shen X, Kong J. Acral melanoma in Chinese: a clinicopathological and prognostic study of 142 cases. Sci Rep. 2016;6:31432.
Cascinelli N, Zurrida S, Galimberti V, Bartoli C, Bufalino R, Del Prato I, et al. Acral lentiginous melanoma. A histological type without prognostic significance. J Dermatol Surg Oncol. 1994;20:817–22.
Castaneda CA, Torres-Cabala C, Castillo M, Villegas V, Casavilca S, Cano L, et al. Tumor infiltrating lymphocytes in acral lentiginous melanoma: a study of a large cohort of cases from Latin America. Clin Transl Oncol. 2017;19:1478–88.
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–80.
Torres-Cabala CA, Wang WL, Trent J, Yang D, Chen S, Galbincea J, et al. Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral-lentiginous/mucosal type. Mod Pathol. 2009;22:1446–56.
Vazquez Vde L, Vicente AL, Carloni A, Berardinelli G, Soares P, Scapulatempo C, et al. Molecular profiling, including TERT promoter mutations, of acral lentiginous melanomas. Melanoma Res. 2016;26:93–9.
Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–6.
Merkel EA, Gerami P. Malignant melanoma of sun-protected sites: a review of clinical, histological, and molecular features. Lab Investig. 2017;97:630–5.
Ramani NS, Aung PP, Gu J, Sfamenos S, Sdringola-Maranga C, Nagarajan P, et al. TERT amplification but not activation of canonical Wnt/β-catenin pathway is involved in acral lentiginous melanoma progression to metastasis. Mod Pathol. 2020 May. https://doi.org/10.1038/s41379-020-0565-5. Online ahead of print.
Acknowledgements
The authors thank Ms. Stephanie Deming, ELS, Senior Scientific Editor, Research Medical Library, The University of Texas MD Anderson Cancer Center, for assistance with editing of this paper.
Funding
PPA, MD, PhD, is supported by a grant from the Melanoma Research Alliance and an Institutional Research Grant from The University of Texas MD Anderson Cancer Center. This work was also supported by the National Cancer Institute under award number P30CA016672, which supports the MD Anderson Cancer Center Clinical Trials Office.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mejbel, H.A., Torres-Cabala, C.A., Milton, D.R. et al. Prognostic significance of acral lentiginous histologic type in T1 melanoma. Mod Pathol 34, 572–583 (2021). https://doi.org/10.1038/s41379-020-0641-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0641-x
This article is cited by
-
Caucasians with acral lentiginous melanoma have the same outcome as patients with stage- and limb-matched superficial spreading melanoma
Journal of Cancer Research and Clinical Oncology (2022)
-
Skin Cancer in People of Color: A Systematic Review
American Journal of Clinical Dermatology (2022)