Abstract
The prognostic impact of tumor-infiltrating lymphocytes (TILs) within invasive lobular carcinoma (ILC) remains to be better characterized. In estrogen receptor (ER)-negative invasive ductal carcinomas of no special type (IDC-NST), TILs are associated with good prognosis. The aim of this study was to examine TILs in ILC, with particular focus on prognostic and clinicopathologic features. A cohort comprising 459 consecutive ILCs diagnosed in a single institution from 2005 to 2008 met the eligibility criteria for this study. The percentage of tumor area occupied by TILs was quantified by two breast pathologists and categorized into three groups: no TILs, ≤5%, >5%. Clinicopathologic features were tested by Fisher’s exact tests or Chi2 tests. Overall survival (OS) and invasive disease-free survival (iDFS) were estimated by Kaplan–Meier and Cox proportional hazard statistics. There were 239 TIL-negative cases, 185 cases with ≤5% TILs, and 35 cases with >5% TILs. TILs were associated with younger age, larger tumors, lymph node involvement, poor Nottingham prognostic index, HER2 amplification, multinucleation, and prominent nucleoli (p < 0.05). Poor OS was significantly associated with increasing TILs in the univariate Cox proportional hazards model (p < 0.001) and Kaplan–Meier estimator (p < 0.05, log-rank test). Similar results were observed for iDFS (p = 0.004 for Cox univariate and p = 0.005 for log-rank test). Notably, TILs can identify a subset of ILC patients with poor OS independently of molecular subtype and lymph node metastases (multivariate Cox, p < 0.001, OS hazard ratio (HR) = 4.38 and HR = 6.15, for ≤5% and >5% TILs, respectively, vs. absence of TILs). Prominent nucleoli was the only nuclear feature associated with poor OS (p = 0.05) and iDFS (p = 0.05) in univariate Cox survival analysis. TILs represent a promising new morphologic biomarker associated with poor outcome of ILC, in contrast with that observed in ER-negative IDC-NST.
Similar content being viewed by others
Introduction
Invasive lobular breast cancer (ILC) represents the second most common histologic type of breast cancer (~15% of all breast cancers), constituting a distinct molecular entity from invasive ductal carcinoma of no special type (IDC-NST) [1]. ILC is characterized by proliferation of non-cohesive cells that infiltrate the mammary gland as single cells or rows of cells with little stromal reaction, making the disease difficult to diagnose [2, 3]. About 85% of ILC show loss of expression of E-cadherin protein, which is involved in cell adhesion, and are predominantly hormone receptor-positive (estrogen (ER) and/or progesterone (PR) receptors) with a low proliferation rate [4]. Current clinical management of ILC consists of surgery and endocrine therapy. Response rates to neoadjuvant chemotherapy are much lower than those observed for IDC-NST [5,6,7,8]. Some studies indicate that ILC patients have a poorer long-term (>15 years) prognosis compared with ER-positive IDC-NST [9,10,11], suggesting that ILC cells demonstrate dormancy. Furthermore, the metastatic pattern of ILC includes bone, skin, and atypical sites such as peritoneum, ovary, and gastrointestinal tract [10, 12]. These clinical specificities constitute a challenge for initial clinical management. New biomarkers that can specifically predict ILC prognosis in order to tailor treatment therefore need to be identified.
Recent studies described the molecular landscape of ILC and defined molecular subgroups [13,14,15]. ILC is characterized by recurrent mutations affecting the CDH1 gene (coding for E-cadherin), PI3K/AKT pathway, ERBB2, and genes involved in hormone response (ESR1, GATA3, FOXA1). Ciriello et al. [13] identified three ILC subtypes in a subset of TCGA cases that they called immune related, reactive like, and proliferative. Reactive-like tumors were associated with a better prognosis compared with the proliferative group [13]. Michaut et al. analyzed the RATHER cohort and identified a hormone-related group characterized by genes and proteins involved in hormonal response, and an immune-related group characterized by immune markers including genes involved in immune checkpoint inhibition [14]. These studies indicate the major role played by the immune response in ILC biology.
Tumor-infiltrating lymphocytes (TILs) are a key component of the adaptive immune system with a crucial impact on cancer progression. In hormone receptor-negative invasive breast tumors, TILs constitute a morphologic marker for predicting response to adjuvant chemotherapy and prognosis in triple-negative breast carcinomas and HER2-positive breast carcinomas [16,17,18,19], while the role of TILs as a prognostic biomarker in ER-positive breast carcinomas remains unclear. Recent evidence suggests that, in ER-positive ILC, TILs may have a potential impact on patient outcome [20]. Further studies are necessary to clarify the impact of TILs and their immunologic characteristics on the prognosis of ILC.
The aim of this study was to characterize TILs in a large cohort of ILC patients followed in a single institution, with particular focus on TIL quantification, the association between TIL and clinical and pathologic features and the intrinsic prognostic value of TIL.
Material and methods
Patients and samples
Patient eligibility criteria for this study were: cases of pure ILC primary breast cancer operated between January 2005 and December 2008, with no neoadjuvant therapy (endocrine or chemotherapy), written patient consent to use their tumor material for research and availability of a tumor block. Patient consent was obtained prospectively before surgery. We retrospectively retrieved 526 cases from the Institut Curie electronic database. All slides were reviewed by experienced breast pathologists (JCT and AVS). Histologic review resulted in a total of 459 patients. Cases were classified into morphologic variants: classic, solid, alveolar, or mixed type of lobular carcinomas when a second component represented more than 10%, according to World Health Organization criteria [4]. Micro-ILC, non-lobular carcinomas, mixed carcinomas with a ductal component >10%, multifocal or multicentric carcinomas with a non-lobular second tumor were excluded after histologic review. Nuclear features such as multinucleation and visible nucleoli at 40×, namely proeminent nucleoli, were registered when present. ILC tumor grade was determined according to the Elston–Ellis grading system [21]. Patient characteristics and outcomes were retrieved from electronic medical files.
Histopathologic analysis of tumor-infiltrating lymphocytes
Stroma TILs were quantified as recommended by Salgado et al. [22]. Briefly, TILs were defined as infiltrating mononuclear inflammatory cells observed within the tumor stroma. Areas of carcinoma in situ, necrosis and crush artifacts were not included. TILs were assessed as a continuous parameter and categorized after analyzing their distribution. In cases harboring TILs (TIL-positive ILCs), the median TILs level was of 5.0%, with an interquartile range of 2.0–5.0%. On the basis of this distribution, ILC cases were categorized as negative (0%), containing ≤5% of TILs and >5% of TILs. Histopathologic examination of TILs was performed by breast pathologists blinded to clinical information, including treatment allocation and outcomes.
Immunostaining and histo-molecular subtyping
All cases included in this study were reviewed for ER, PR, HER2, and E-cadherin (if performed during the initial diagnostic procedure). Further immunostainings were performed when initially missing or when considered necessary. In brief, immunostaining was performed on 4 µm tissue sections prepared from a representative sample of the tumor. After rehydration and antigen retrieval in citrate buffer (10 mM, pH 6.1), tissue sections were stained for ER (clone 6F11, Novocastra, 1/200), PR (clone 1A6, Novocastra, 1/200), and ERBB2 (CB11, Leica Biosystems, 1/100) and E-cadherin when necessary to confirm ILC diagnosis (clone HECD1, Zymed Laboratories Inc, 1/50). Staining reaction was performed with the Vectastain Elite ABC peroxidase mouse IgG kit (Vector Burlingame, CA) and diaminobenzidine (Dako A/S, Glostrup, Denmark) as chromogen. Positive and negative controls were included in each run. Cases were considered positive for ER and PR according to standardized guidelines using a percentage of 10% of positive nuclei [23]. A tumor was considered HER2-positive by immunohistochemistry when it scored 3+ with uniform intense membrane staining of more than 10% of invasive tumor cells. These cases were confirmed to harbor ERBB2 gene amplification by FISH according to ASCO/CAP guidelines [24]. ILCs scored 2+ by HER2 immunohistochemistry were considered to be HER2-positive when FISH test positive. Histo-molecular subtypes were defined as follows: luminal A—ER and/or PR ≥ 10% and mitotic score of 1; luminal B—ER ≥ 10%, PR < 20% or mitotic score of 2 or 3; HER2—HER2-positive; triple-negative—ER-negative (<10%), PR-negative (<10%), and HER2 not amplified [24,25,26]. A cut-off of 10% for ER/PR positivity was used in accordance with GEFPICS guidelines [27, 28] and studies indicating that ER < 10% cases have a similar clinical behavior to that of ER-negative cases [26, 29,30,31,32,33,34].
Statistical analyses
Baseline characteristics were summarized as frequency and percentage for qualitative data and mean and standard deviation or median with minimum and maximum for continuous variables. Associations between categorical variables were evaluated using Chi2 or Fisher’s exact test.
Overall survival (OS) was defined as the time from diagnosis of breast cancer until the date of death from any cause. Patients still alive were censored at the date of last news. Invasive disease-free survival (iDFS) was defined as the interval from diagnosis of breast cancer until the first breast cancer event including local, regional, distant recurrence, contralateral disease, second non-breast primary cancer, and mortality from any cause. OS and iDFS were estimated using the Kaplan–Meier method and comparisons between TILs subgroups were performed with the log-rank test. Univariate and multivariate Cox regression models were carried out to evaluate prognosis. To assess the relative influence of prognostic factors using the multivariate Cox stepwise procedure, the inclusion criterion was p ≤ 0.2 and the exclusion criterion was p > 0.2 for covariates. For all analyses, a p value ≤ 0.05 was considered to be statistically significant.
Results
Clinical and pathologic data
We retrospectively retrieved 526 consecutive ILCs satisfying the eligibility criteria. After histologic review, 67 (13%) carcinomas were excluded (15 non-ILC, 49 carcinomas with a ductal component representing more than 10%, 3 multifocal or multicentric carcinomas with a non-lobular invasive second tumor), resulting in a total of 459 cases included in the analysis. The median age of these patients at diagnosis was 60 years. The majority of patients were postmenopausal women (77.7%—N = 330), with unifocal disease (83.8%—N = 378). Surgical treatment comprised lumpectomy for 69.9% of patients (N = 321) and mastectomy for 30.1% of patients (N = 138). The majority of ILCs were of classic subtype (68%—N = 312), followed by mixed subtype (26%—N = 119), alveolar subtype (3.7%—N = 11), and solid subtype (2.4%—N = 11). Cases were classified into histologic grades 1, 2, and 3 in 9.8% (N = 45), 79.7% (N = 366), and 10.5% (N = 48) of ILCs, respectively. The majority of ILCs had a tumor size ≤ 2 cm (67.3% for pT1—N = 309) and were lymph node-negative (74.1%—N = 340). The Nottingham Prognostic Index (NPI) was excellent/good in 54.8% (N = 249) of ILC patients and moderate/poor in 45.2% (N = 206) of cases. Multinucleated cells, mostly binucleated cells, were found in 26.4% (N = 121) of ILCs and proeminent nucleoli were present in 25.6% (N = 116) of cases.
Postsurgical treatment of patients consisted of standard treatment: endocrine therapy was administered to hormone receptor-positive patients (79.7%—N = 366) and endocrine therapy and/or trastuzumab was administered to HER2-positive patients (2.4%—N = 13). Adjuvant chemotherapy was administered to 21.8% (N = 100) of ILC patients and radiotherapy was administered to 91.3% (N = 419) of ILC patients. Median patient follow-up was 106 months (range: 3–156 months), with 37 deaths due to disease, 22 local recurrences, 4 lymph node metastases, and 41 distant metastases (Table 1).
ILC phenotype
The majority of cases expressed hormone receptors: 96.1% (N = 441) of ILCs were ER-positive and 72.5% (N = 332) were PR-positive. HER2 amplification/overexpression was demonstrated in 18 patients (4.4%—N = 18) (Table 1). The majority of ILCs were clinically defined as luminal A, corresponding to 55.5% (N = 254) of all cases of the series, followed by 37.8% (N = 173) of luminal B subtype, 3.9% (N = 18) of HER2-positive subtype, and 2.8% (N = 13) of triple-negative subtype. ILC molecular subtypes were significantly associated with histologic variants (p = 0.003). More specifically, luminal B ILCs encompassed many mixed (47.1%—56/119) and solid carcinomas (72.7%—8/11). There were no HER2-positive or triple-negative alveolar ILCs (Table 2).
TILs quantification in ILC
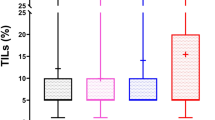
ILCs were characterized by low lymphocyte infiltration, as the mean TIL value was 2.7% for all cases. The mean TIL score for TIL-positive ILCs was 5.6%, with a median of 5.0%, and an interquartile range of 2.0–5.0%. The majority of ILCs were classified as TIL-negative, as TILs were not detected in 239 carcinomas (52.1%—N = 239) (Table 3 and Fig. 1a), 185 ILCs (40.3%) showed ≤5% of TILs and 35 ILCs (7.6%—N = 359) presented >5% of TILs (Table 3 and Fig. 1b). Lymphocytes were highly predominant (≥40%) in only four cases of ILC.
Association between TILs and clinicopathologic features
Compared with TIL-negative ILCs, TIL-positive ILCs (≤5% and >5%) were significantly associated with lower age at diagnosis (p = 0.025), histologic grade (p = 0.002), tumor size (p = 0.036), and lymph node metastasis (p = 0.003) and poor NPI (p = 0.001) (Table 3). Of note, TILs > 5% ILCs were associated with multinucleation (p < 0.001) and the presence of prominent nucleoli (p = 0.001) (Fig. 2a, b, respectively, and Table 3). The TIL-positive ILC subgroup also presented a higher rate of HER2 amplification/overexpression (p = 0.004) and an association with HER2-positive molecular subtype. No significant association was observed between the presence of TILs and ER or PR expression (p < 0.05, not shown). The majority of alveolar ILCs were TIL-negative (14 out of 17 cases (82.4%)). In contrast, we found a small but statistically significant TIL enrichment in mixed ILC (34.3% of carcinomas with >5% TILs were mixed ILC vs. 23.8% of TIL-negative carcinomas were mixed ILC) (Table 3).
Association between TILs and prognosis
iDFS and OS were significantly different in the various TIL subgroups. More specifically, an increasing TIL score among ILC was associated with progressively poorer iDFS and OS on Kaplan–Meier analysis (log-rank test, p = 0.005 for iDFS and p = 0.00031 for OS) (Fig. 3a, b, respectively). Ten-year OS in the TIL-negative subgroup was 95.9% (95% CI: 92.6–99.4%), in contrast with 82.0% (95% CI: 69.9–96.2%) for the TILs > 5% subgroup, corresponding to a 13.9% survival difference. An identical result was observed for iDFS (Supplementary Table 1).
Univariate Cox proportional hazards analysis showed an increased risk of relapse with increasing TIL scores (hazard ratio (HR) = 1.93 and HR = 2.93 in the groups containing ≤5% TILs and >5% TILs, respectively, p < 0.004). Similarly, an association was observed between the risk of death with increasing TIL scores (HR = 4.12 and HR = 5.31, in the groups containing ≤5% TILs and >5% TILs, respectively, p < 0.001). Univariate analysis showed that the presence of lymph node metastasis alone was associated with poorer iDFS and OS (p = 0.003 and p = 0.001, respectively). Univariate analysis also showed that molecular subtype was significantly associated with OS. More specifically, luminal B and triple-negative carcinomas presented a higher risk of death from breast cancer than luminal A ILC (HR = 2.99, 95% CI: 1.53–6.12 and HR = 3.81, 95% CI: 0.74–12.77, respectively). HER2 overexpressing ILC treated by adjuvant chemotherapy plus anti-HER2 therapy (13 out of 18 cases) showed the lowest risk of death (HR = 0.53, 95% CI: 0.004–4.02). The presence of prominent nucleoli was significantly associated with poor iDFS and poor OS (p = 0.05 and p = 0.049, respectively) (Table 4).
To determine whether the prognostic impact of TILs was associated with HER2-positive and TNBC molecular subtypes, which are known to be associated with poor prognosis in IDC-NST, we performed univariate Cox proportional hazard analysis excluding these two molecular subtypes. We found that the prognostic impact of TILs on iDFS and OS was maintained after excluding TNBC and/or HER2-positive cases, with comparable HRs (Supplementary Table 2).
To determine whether the contribution of TILs to poor prognosis was associated with lymph node metastasis, we stratified univariate Cox analysis into node-negative and node-positive (N1, N2, N3) subgroups. Notably, the lymph node involvement was not different among ER+ and ER− ILCs (Supplementary Table 3a). Survival analysis considering only node-negative patients (N = 340) showed a statistically significant prognostic impact of TIL levels (p = 0.001 for iDFS and p = 0.003 for OS). Similarly, when only node-positive patients (N = 115) were considered, we observed an identical significant impact of TIL levels on OS (p = 0.054) (Supplementary Table 3b). We also did not observe any association between lymph node metastasis and molecular subtypes in our ILC series (p < 0.05, data not shown).
Multivariate survival analysis showed that the impact of TILs on OS and iDFS was independent of the number of metastatic lymph nodes and the molecular subtype of ILC. HR values were 6.15 and 3.47 in the TILs > 5% ILCs for OS and iDFS models, respectively (p < 0.001 and p = 0.001), in contrast with the lower HR values found in univariate models (Table 4).
Discussion
Our study comprehensively addressed the prognostic impact of TILs in a large cohort of primary surgically treated ILCs with follow-up from a single institution. We revealed that increased TILs were associated with poor OS and poor iDFS in univariate and multivariate models. Furthermore, our study showed the importance of TILs to refine ILC prognosis by demonstrating that the association between TILs and poor iDFS and poor OS was independent of lymph node metastases and ILCs molecular subtypes in multivariate analysis.
Desmedt et al. showed that, in ILC patients, TILs enrichment was associated with young age, lymph node involvement, high proliferative tumors and poorer prognosis in univariate models, but multivariate analysis of breast cancer-free survival adjusted for standard clinicopathologic variables resulted in ambiguous results with a prognostic impact of TILs in the group with intermediate TILs, but no significant impact in the group with high TILs [20].
We confirmed that ILCs are characterized by low levels of TILs. We also found that TILs were significantly associated with higher grade carcinomas, tumor size, lymph node metastases, and NPI index, suggesting a pro-tumorigenic role of TILs in ILC that deserves further investigation. The presence of prominent nucleoli and multinucleated cells, which are associated with more aggressive tumor behavior in breast cancers and in other tumor types [35], was significantly associated with TILs. These nuclear features were identified during retrospective analysis of ILC cases and could potentially be combined with other pathologic parameters to more accurately classify ILCs in clinical practice. In addition, like Desmedt et al. [20], we found an association between lymphocyte infiltration and histologic ILC variants, as the alveolar histotype was more often TIL-negative, while mixed ILC carcinomas were mostly TIL-enriched. These observations are consistent with the favorable outcome reported for alveolar ILC and the poor outcome described for mixed ILC [36,37,38]. The immunologic implications of these observations need to be investigated.
The presence of high levels of TILs has been consistently associated with a more favorable prognosis in patients with early stage triple-negative and HER2-positive breast cancer [16,17,18,19, 39, 40]. Hormone receptor-positive breast carcinomas have been recognized to harbor low levels of TILs [41], the prognostic impact of which remains uncertain [41,42,43]. Our observation that TILs are associated with poor outcome in ILC therefore provides new insight into the role of immune cells in hormone receptor-positive breast carcinoma. Of note, our series of ILC comprised a low frequency of triple-negative and HER2-positive subtypes, but we decided to include these cases in our analysis because they reflect the true heterogeneity of ILC in the clinical setting. In addition, in our study composed of consecutive ILC patients treated in a single institution, we found a higher proportion of luminal B subtype, in accordance with Iorfida et al. [37], compared with that observed in other ILC studies composed of selected cases for molecular analyses [13,14,15].
HER2 amplification/overexpression was rarely observed in our ILC series, representing only 4.4% (18/405) of ILCs (Table 1), in accordance with other studies [37]. We found that HER2-positive ILCs were associated with a low risk of cancer death, reflecting the contribution of trastuzumab therapy to the improved survival of ILC, as shown in the HERA trial [44]. Da Ros et al. also demonstrated that HER2-positive ILCs presented lower proliferative activity and lower histologic grade compared with HER2-positive IDC-NST [45]. In our study, we found that HER2-positive ILC were slightly but significantly enriched in TILs. The exact nature of these TILs is unknown and their role or interaction with anti-HER2 therapy needs to be elucidated.
Newly defined molecular subtypes of ILC have been proposed [13, 14]. Michaut et al. reported an immune-related subgroup of ILCs that is enriched in TILs, associated with lower expression of ER and PR genes, higher cytokine and chemokine signaling and higher expression of suppressive immune molecules PDL1, PD1, and CTLA4 [14]. Ciriello et al. independently described a different immune-related subgroup of ILCs characterized by expression of modulators of immunogenic signaling including cytokines, chemokines, and major histocompatibility complex [13]. Of note, the prognostic significance of the classifications reported by these two teams is not clear and the degree of overlap between these groups appears to be low. Further studies on our ILC series are necessary to clarify the association between TIL-enriched ILC and the molecular subgroups of ILC, as transcriptomic studies and assessment of the detailed immune composition in terms of the origin and levels of immune cells are ongoing and will provide greater insight into the clinical and biologic behavior of the tumors analyzed in this study.
This study has a number of limitations. It is a retrospective study and we cannot rule out heterogeneity between our cohort and the cohorts of other studies [20]. The small sample size of HER2-positive and TNBC groups limits analysis of these subgroups in the context of ILC. Complete phenotypic characterization of immune cells in TIL-positive cases and molecular characterization of all ILC patients are necessary in order to elucidate the mechanisms responsible for the observed aggressive behavior of TIL-enriched ILC. As ILC patients have few targeted treatment options, a better understanding of the immune composition and transcriptomic heterogeneity of ILC could help to identify ILC as a distinct malignancy that needs to be treated as a separate clinical entity.
In conclusion, this study found that TIL assessment in ILC contributes valuable prognostic information, as it was associated with poor outcome in a large cohort of patients followed in a single cancer center. The prognostic value of TIL was shown to be independent of molecular subtypes and lymph node metastases. For the first time, we found an association between TILs and aggressive nuclear features.
References
Gruel N, Lucchesi C, Raynal V, Rodrigues MJ, Pierron G, Goudefroye R, et al. Lobular invasive carcinoma of the breast is a molecular entity distinct from luminal invasive ductal carcinoma. Eur J Cancer. 2010;46:2399–407.
Sikora MJ, Jankowitz RC, Dabbs DJ, Oesterreich S. Invasive lobular carcinoma of the breast: patient response to systemic endocrine therapy and hormone response in model systems. Steroids. 2013;78:568–75.
Guiu S, Wolfer A, Jacot W, Fumoleau P, Romieu G, Bonnetain F, et al. Invasive lobular breast cancer and its variants: how special are they for systemic therapy decisions? Crit Rev Oncol Hematol. 2014;92:235–57.
World Health Organization Classification of Tumours Editorial Board. Breast tumours. 5th ed. Vol. 2. Lyon: International Agency for Research on Cancer; 2019.
Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau SW, Broglio K, Theriault RL, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23:41–8.
Tubiana-Hulin M, Stevens D, Lasry S, Guinebretiere JM, Bouita L, Cohen-Solal C, et al. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006;17:1228–33.
Bollet MA, Savignoni A, Pierga JY, Lae M, Fourchotte V, Kirova YM, et al. High rates of breast conservation for large ductal and lobular invasive carcinomas combining multimodality strategies. Br J Cancer. 2008;98:734–41.
Mathieu MC, Rouzier R, Llombart-Cussac A, Sideris L, Koscielny S, Travagli JP, et al. The poor responsiveness of infiltrating lobular breast carcinomas to neoadjuvant chemotherapy can be explained by their biological profile. Eur J Cancer. 2004;40:342–51.
Adachi Y, Ishiguro J, Kotani H, Hisada T, Ichikawa M, Gondo N, et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer. 2016;16:248.
Korhonen T, Kuukasjarvi T, Huhtala H, Alarmo EL, Holli K, Kallioniemi A, et al. The impact of lobular and ductal breast cancer histology on the metastatic behavior and long term survival of breast cancer patients. Breast. 2013;22:1119–24.
Pestalozzi BC, Zahrieh D, Mallon E, Gusterson BA, Price KN, Gelber RD, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26:3006–14.
Ferlicot S, Vincent-Salomon A, Medioni J, Genin P, Rosty C, Sigal-Zafrani B, et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer. 2004;40:336–41.
Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive molecular portraits of invasive lobular breast. Cancer Cell. 2015;163:506–19.
Michaut M, Chin SF, Majewski I, Severson TM, Bismeijer T, de Koning L, et al. Integration of genomic, transcriptomic and proteomic data identifies two biologically distinct subtypes of invasive lobular breast cancer. Sci Rep. 2016;6:18517.
Desmedt C, Zoppoli G, Gundem G, Pruneri G, Larsimont D, Fornili M, et al. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol. 2016;34:1872–81.
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66.
Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25:1536–43.
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50.
Pruneri G, Vingiani A, Denkert C. Tumor infiltrating lymphocytes in early breast cancer. Breast. 2018;37:207–14.
Desmedt C, Salgado R, Fornili M, Pruneri G, Van den Eynden G, Zoppoli G, et al. Immune infiltration in invasive lobular breast cancer. J Natl Cancer Inst. 2018;110:768–76.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71.
MacGrogan G, Mathieu MC, Poulet B, Penault-Llorca F, Vincent-Salomon A, Roger P, et al. [Pre-analytical stage for biomarkerassessment in breast cancer: 2014 update of the GEFPICS' guidelines in France]. Ann Pathol. 2014;34:366–72.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105–22.
Amin MB. AJCC cancer staging manual. 8th ed. Chicago, Illinois, Springer; 2018.
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–66
Balaton AL, Coindre JM, Collin F, Ettore F, Fiche M, Jacquemier J, et al. [Recommendations for the immunohistochemical evaluation of hormone receptors on paraffin sections of breast cancer. Study group on hormone receptors using immunohistochemistry FNCLCC/AFAQAP. National Federation of Centres to Combat Cancer/French Association for Quality Assurance in Pathology]. Ann Pathol. 1996;16:144–8.
Zafrani B, Aubriot MH, Mouret E, De Cremoux P, De Rycke Y, Nicolas A, et al. High sensitivity and specificity of immunohistochemistry for the detection of hormone receptors in breast carcinoma: comparison with biochemical determination in a prospective study of 793 cases. Histopathology. 2000;37:536–45.
Balduzzi A, Bagnardi V, Rotmensz N, Dellapasqua S, Montagna E, Cardillo A, et al. Survival outcomes in breast cancer patients with low estrogen/progesterone receptor expression. Clin Breast Cancer. 2014;14:258–64.
Chen T, Zhang N, Moran MS, Su P, Haffty BG, Yang Q. Borderline ER-positive primary breast cancer gains no significant survival benefit from endocrine therapy: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:1–8.
Deyarmin B, Kane JL, Valente AL, van Laar R, Gallagher C, Shriver CD, et al. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. 2013;20:87–93.
Early Breast Cancer Trialists’ Collaborative Group, Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84.
Gloyeske NC, Dabbs DJ, Bhargava R. Low ER+ breast cancer: is this a distinct group? Am J Clin Pathol. 2014;141:697–701.
Raghav KP, Hernandez-Aya LF, Lei X, Chavez-Macgregor M, Meric-Bernstam F, Buchholz TA, et al. Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer. 2012;118:1498–506.
Elsharawy KA, Toss MS, Abuelmaaty SR, Ball G, Green AR, Aleskandarany MA, et al. Prognostic Significance of nucleolar assessment in invasive breast cancer. Histopathology. 2020;76:671–84
du Toit RS, Locker AP, Ellis IO, Elston CW, Nicholson RI, Blamey RW. Invasive lobular carcinomas of the breast–the prognosis of histopathological subtypes. Br J Cancer. 1989;60:605–9.
Iorfida M, Maiorano E, Orvieto E, Maisonneuve P, Bottiglieri L, Rotmensz N, et al. Invasive lobular breast cancer: subtypes and outcome. Breast Cancer Res Treat. 2012;133:713–23.
Shousha S, Backhous CM, Alaghband-Zadeh J, Burn I. Alveolar variant of invasive lobular carcinoma of the breast. A tumor rich in estrogen receptors. Am J Clin Pathol. 1986;85:1–5.
Park JH, Jonas SF, Bataillon G, Criscitiello C, Salgado R, Loi S, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol. 2019;30:1941–49
Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37:559–69.
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7.
Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228–41.
Metzger-Filho O, Procter M, de Azambuja E, Leyland-Jones B, Gelber RD, Dowsett M, et al. Magnitude of trastuzumab benefit in patients with HER2-positive, invasive lobular breast carcinoma: results from the HERA trial. J Clin Oncol. 2013;31:1954–60.
Da Ros L, Moretti A, Querzoli P, Pedriali M, Lupini L, Bassi C, et al. HER2-positive lobular versus ductal carcinoma of the breast: pattern of first recurrence and molecular insights. Clin Breast Cancer. 2018;18:e1133–9.
Acknowledgements
The authors would like to thank Martial Caly for his scientific support, the SIRIC 2 for logistic and technical support, and the European Lobular Breast Cancer Consortium (ELBCC).
Funding
AFV received support from a Mayent-Rothschild fellowship from Institut Curie, France and IPATIMUP/Fundação para a Ciência e Tecnologia, Portugal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the local ethics committee (Institut Curie Breast Cancer Group).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tille, JC., Vieira, A.F., Saint-Martin, C. et al. Tumor-infiltrating lymphocytes are associated with poor prognosis in invasive lobular breast carcinoma. Mod Pathol 33, 2198–2207 (2020). https://doi.org/10.1038/s41379-020-0561-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0561-9
This article is cited by
-
Deep learning model improves tumor-infiltrating lymphocyte evaluation and therapeutic response prediction in breast cancer
npj Breast Cancer (2023)
-
Clinical outcomes and prognostic factors in triple-negative invasive lobular carcinoma of the breast
Breast Cancer Research and Treatment (2023)
-
Biomarker profile of invasive lobular carcinoma: pleomorphic versus classic subtypes, clinicopathological characteristics and prognosis analyses
Breast Cancer Research and Treatment (2022)
-
Invasive lobular carcinoma of the breast: the increasing importance of this special subtype
Breast Cancer Research (2021)
-
The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group
npj Breast Cancer (2021)