Abstract
Prostatic adenocarcinoma and urothelial carcinoma typically demonstrate distinct morphologic and immunohistochemical features. However, high-grade prostate and urothelial carcinomas sometimes show significant morphologic and immunohistochemical overlap, which can result in misdiagnosis and mistreatment. This diagnostic dilemma is particularly acute in patients previously treated with radiation and/or hormone therapy for prostate cancer, who later present with high-grade carcinoma in the urinary bladder. To address the diagnostic utility of integrated immunohistochemical and molecular analysis in this setting, we evaluated 25 high-grade carcinomas of the bladder for which morphologic features were deemed indeterminate. Our analysis included immunohistochemistry for urothelial markers (GATA3, p63, uroplakin II), prostate markers (NKX3.1, prostate specific antigen, P501S), androgen receptor (AR) and ERG, along with molecular characterization using capture-based next generation DNA sequencing. Immunohistochemical findings were concordant with the final integrated diagnosis in 21 (84%) cases. However, in three (12%) cases, immunohistochemistry supported a diagnosis of urothelial carcinoma, but molecular analysis identified the correct diagnosis of prostate cancer based on the presence of a TMPRSS2-ERG fusion. One case remained unclassifiable even after this integrated analysis. Notably, in 1 of 21 cases, the presence of a TERT promoter mutation and the absence of a TMPRSS2-ERG fusion would typically favor a diagnosis of urothelial carcinoma, but the aggregate immunohistochemical and molecular findings instead supported a diagnosis of microsatellite unstable prostatic adenocarcinoma with deep deletion of MSH2 and MSH6. Our findings highlight the importance of considering prostatic origin in high-grade carcinoma of the urinary bladder of patients with a history of treated prostate cancer, even when the immunohistochemical findings favor urothelial carcinoma. In a subset of cases, an approach that integrates immunophenotypic and molecular data may help correctly assign site of origin and prevent misdiagnosis that can result from overreliance on any individual immunohistochemical or molecular result.
Similar content being viewed by others
Introduction
Prostatic adenocarcinoma (PCa) and urothelial carcinoma (UCa) are typically readily distinguishable based on their unique morphologic and immunohistochemical features. PCa usually demonstrates glands composed of cells with relatively monomorphic nuclei and prominent nucleoli, while UCa are generally characterized by nests and sheets of tumor cells with pleomorphic nuclei. There are multiple sensitive and specific immunohistochemical stains available to assist in diagnosis when needed. PSA, NKX3.1, and P501S demonstrate high sensitivity and specificity for PCa [1, 2] while GATA3, p63, and uroplakin II demonstrate good sensitivity for UCa and are highly specific for excluding PCa [3, 4].
High-grade PCa can adopt architectural and cytologic features that overlap with UCa, potentially leading to misdiagnosis. These features include sheet-like and pseudopapillary growth [5], squamous differentiation [6], and increased nuclear pleomorphism and mitotic rate. In addition, UCa can adopt PCa-like glandular differentiation, further complicating distinction. While immunohistochemistry is often helpful in cases of morphologic overlap, our experience is that high-grade PCa sometimes demonstrates unexpected and misleading immunohistochemical features, including loss of expected prostate markers and aberrant expression of proteins typically seen in UCa. This phenomenon has been reported by others, especially in cases of PCa with squamous or neuroendocrine differentiation [2, 6,7,8]. In cases lacking any conventional morphologic or immunohistochemical features, high-grade PCa cannot be reliably distinguished from UCa despite careful analysis, and we occasionally resort to a descriptive diagnosis of “high-grade carcinoma” with a comment indicating that the tumor could be of urothelial or prostatic origin.
This diagnostic dilemma is particularly acute in patients with a history of PCa treated with radiation and/or hormone therapy who subsequently present with a high-grade carcinoma in the urinary bladder. The history of PCa, particularly if remote, may also be unknown to the pathologist evaluating a specimen for a urinary bladder mass. PCa recurrences after radiation frequently show high-grade features [9,10,11], increasing the probability of morphologic overlap with UCa. These patients may also have a modestly increased risk of a secondary UCa [12, 13], although this remains controversial. The result is a diagnostic dilemma that may not be resolved through morphologic and immunohistochemical evaluation: is this tumor in the bladder a high-grade PCa recurrence or is it instead a new UCa? Accurate distinction of these entities is essential, as UCa and PCa have very different prognostic and therapeutic implications.
UCa and PCa demonstrate characteristic molecular findings that may assist in diagnosis [14,15,16]. Chromosomal translocations that result in fusion of the androgen-regulated TMPRSS2 gene with an Ets family transcription factor (most commonly ERG) are seen in roughly half of PCa [14], and based on a review of the published literature and the cBioportal database [17, 18], we know of no confirmed example of a TMPRSS2 fusion in a UCa. Therefore, in a patient with a history of PCa who presents with a new high-grade carcinoma in the bladder, we consider a TMPRSS2 fusion as pathognomonic of prostatic origin. Conversely, hotspot TERT promoter mutations that generate a binding site for the Ets family transcription factor GABPA/Ets1 are seen in ~70% of UCa but are exceedingly rare in PCa based on the published literature and the cBioPortal database [19, 20]. Despite their known lack of sensitivity, the specificity of TMPRSS2 fusions and TERT promoter mutations for PCa and UCa, respectively, has led to use of these two molecular markers to provide evidence for prostatic or urothelial origin, which has been described by multiple research groups [7, 21,22,23,24].
In addition to TMPRSS2 fusions and TERT promoter mutations, other molecular alterations can provide evidence for prostatic or urothelial origin. In particular, the chromatin modifier genes ARID1A, KMT2D, and KDM6A are among the most commonly mutated genes in UCa [15, 16], and based on cBioportal data, ARID1A, KMT2D, and KDM6A mutations are much more common in UCa (~25%, 25%, and 30% of tumors, respectively) than in PCa (~1%, 6%, and 2% of tumors, respectively). Conversely, AR amplification and PTEN deep deletion are much more common in PCa (~20% and 15% of tumors, respectively) than in UCa (~0.2% and 2% of tumors, respectively), based on cBioportal data. Although these alterations are not as specific as TMPRSS2 fusions and TERT promoter mutations for prostatic versus urothelial origin, their presence can nevertheless assist in distinguishing PCa from UCa in some cases.
Here, we report the utility of integrated immunohistochemical and molecular analysis for diagnosis of bladder masses in a cohort of patients previously treated for PCa; and we highlight the pitfalls of relying on isolated immunohistochemical or molecular findings in this challenging clinical context.
Materials and methods
Case selection and histopathology
UCSF IRB approval was obtained. The UCSF Pathology archives spanning the years 2007–2019 was searched for patients who had undergone resection (transurethral or cystectomy/cysto-prostatectomy) for a urinary bladder mass in which prior radiation therapy for PCa was documented in the clinical history. Relevant clinical history was obtained from the electronic medical record. Slide review was performed by two pathologists who sign out genitourinary pathology routinely (EC and KG) and cases of morphologically high-grade carcinomas that were not obviously PCa by morphology were selected for further study. Cases containing areas of morphologically overt PCa (no adjunct immunohistochemical staining required to reach the diagnosis) were excluded. Specific morphologic features seen in selected cases (listed in Fig. 1) were recorded as present or absent.
a Shows original diagnosis, patient age at time of evaluation for the urinary bladder mass, years elapsed since initial radiation for PCa and additional morphologic findings (black fill = present, white fill = absent). b Shows immunohistochemical staining results for each case (‘−’ = no staining, ‘+’ = weak staining in <50% of the tumor cells, ‘++’ = patchy strong staining in <50% of the tumor cells, ‘+++’ = diffuse strong staining in >50% of the tumor cells; orange highlight = positive marker favors UCa, blue highlight = positive marker favors PCa, ‘NA’ = no tissue available). c Provides a summary of key molecular alterations identified (black fill = present, white fill = absent; genomic alteration type: T = truncating (nonsense, frameshift), D = deep deletion, A = amplification, I = in-frame deletion; orange highlight = alteration present favors UCa, blue highlight = alteration present favors PCa). Please refer to Supplemental Fig. 1 and Supplemental Table 2 for all pathogenic and likely pathogenic molecular alterations identified in each case. d Shows our overall immunohistochemical and molecular impressions if based on the findings in b and c alone, and integrated overall impression (orange fill = UCa, blue fill = PCa, purple fill = HGNOS).
Immunohistochemistry
The following antibodies were used and run on a Ventana Benchmark: Androgen receptor-64 (SP107, RTU, Cell Marque), ERG-1 (EPR3864, RTU, Ventana), and GATA3 (L50-823, RTU, Ventana); and the following antibodies were used and run on the Leica BOND III platform: NKX3.1 (EP356, undiluted, Cell Marque), PSA (ER-PR8, 1:500, DAKO), P501S (1OE3, 1:200, DAKO), p63 (4A4, undiluted, Ventana), uroplakin II (BC21, undiluted, BioCare). Immunohistochemistry was scored as negative (no staining), + (weak staining in <50% of the tumor cells), ++ (patchy strong staining in <50% of the tumor cells), or +++ (diffuse strong staining in >50% of the tumor cells). GATA3, p63, and uroplakin were considered as urothelial markers and NKX3.1, PSA, and P501S were considered as prostate markers.
Capture-based next generation DNA sequencing
Sequencing libraries were prepared from genomic DNA extracted from punch biopsies or macrodissected unstained sections from formalin-fixed, paraffin-embedded tissue containing tumor; and sequencing was performed on an Illumina HiSeq 2500 (San Diego, CA, USA) using an assay that targets the coding regions of 479 cancer-related genes, select introns from 41 genes, and the TERT promoter with a total sequencing footprint of 2.8 Mb, as previously described [25], which has been validated by the UCSF Clinical Cancer Genomics Laboratory. Molecular analysis was performed blinded to the original diagnosis and immunohistochemical impression (BS). Somatic single nucleotide variants and indels were visualized and verified using the Integrative Genomics Viewer. Genome-wide copy number analysis based on on-target and off-target reads was performed by CNVkit and Nexus Copy Number (Biodiscovery, Hawthorne, CA). Molecular alterations were manually classified as pathogenic or likely pathogenic based on information from the following databases: cBioPortal for Cancer Genomics (www.cbioportal.com), ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), and PubMed (https://www.ncbi.nlm.nih.gov/pubmed/). The bioinformatics pipeline included Delly software for detection of structural variants [26], and for all detected fusions, the reads spanning the breakpoints were reviewed by a molecular pathologist using the Integrative Genomics Viewer to confirm the fusion. With respect to detection of TMPRSS2-ERG fusions, the targeted sequencing panel covers all of the exons from both genes, as well as introns 1 and 2 of TMPRSS2.
Results
Twenty-five cases were selected for this study (12 transurethral resections and 13 cystectomies/cysto-prostatectomies). Clinical characteristics and original diagnoses are detailed in Fig. 1a Panel A and Supplemental Table 1. The average years elapsed since initial radiation for PCa was 7 years (range 1–15 years). The original diagnoses included UCa (16/25), PCa (6/25), and high-grade carcinoma not otherwise specified (HGNOS) (3/25). While all tumors showed growth as infiltrating solid sheets and nests of tumor cells, other morphologic features seen were also documented (Fig. 1 Panel A, with pictorial examples shown in Fig. 2a–h). A subset of tumors showed areas of squamous (6/25) or glandular (6/25) differentiation. Five cases showed areas with neuroendocrine features or differentiation, supported by immunohistochemical stains performed on initial clinical workup of these cases. None of the cases contained pleomorphic giant cell morphology. While the majority of cases lacked an obvious in situ component for UCa, papillary/pseudopapillary growth or areas suggestive of or mimicking an in situ component were noted in nine cases (9/25). The presence or absence of other morphologic features commonly used in the differential for UCa versus PCa (cytoplasmic quality, nuclear pleomorphism, nucleoli) for each case are further detailed (Fig. 1 Panel A). The immunohistochemical and molecular findings, as well as our immunohistochemical, molecular and integrated overall impressions for each case (UCa versus PCa) are shown in Fig. 1 Panels B–D. See Supplemental Fig. 1 and Supplemental Table 2 for comprehensive list of pathogenic and likely pathogenic alterations in all 25 cases.
All cases showed areas of solid growth which were considered indeterminate for UCa versus PCa (a, b). Some tumors showed focal areas of glandular (c) and squamous (d) differentiation. Cases often lacked an in situ component (e) or had questionable in situ component (f). Some case showed papillary/pseudopapillary growth (g) and necrosis mimicking papillary growth (h).
UCa cases with concordant immunohistochemical and molecular impressions
In 15 cases (cases 1–14 and 23), both the immunohistochemical and molecular features supported the correct diagnosis of UCa. In these cases, either GATA3 and/or p63 showed significant positive staining (++ or higher, with one case showing weak positive staining (+) for both GATA3 and p63), and all the prostate immunohistochemical markers were negative. P63 was most commonly positive (++ or higher in 87% of cases) and GATA3 and uroplakin were positive in less than half of cases (each ++ or higher in 47% of cases). On molecular analysis, the vast majority of these cases (13/15, 87%) were found to have a hotspot TERT promoter mutation, further supporting UCa. In the two UCa cases lacking a TERT promoter mutation, the molecular impression of UCa was supported by the presence of truncating alterations in ARID1A and at least one other chromatin modifier gene (see Supplemental Fig. 1 and Supplemental Table 2) [15, 16], along with the absence of a TMPRSS2 rearrangement. Of these 15 cases with concordant immunohistochemical and molecular impressions for UCa,14 cases were originally diagnosed as UCa (cases 1–14); and in the remaining case, the integrated studies were able to help further classify a HGNOS case as UCa (case 23).
PCa cases with concordant immunohistochemical and molecular impressions
In six cases (cases 17–22), both the immunohistochemical and molecular findings supported the correct diagnosis of PCa. Five of these cases (cases 17, 19–22) showed strong positive staining (+++) for at least one prostate marker and negative staining for all urothelial markers. The remaining case (case 18) showed focal strong positive staining for p63 (++), but the immunohistochemical impression was nevertheless considered supportive of PCa based on focal strong positive staining for all three prostate markers. On molecular analysis, two of these cases (cases 17 and 18) demonstrated a TMPRSS2-ERG fusion, confirming the diagnosis of PCa. A third case (case 19) demonstrated a TMPRSS2 rearrangement with breakpoint in exon 1 of the gene, but a fusion partner was not identified. This result is nevertheless considered supportive of PCa, as the TMPRSS2 fusion partner is occasionally missed due to the limitations of short-read DNA sequencing analysis for fusion detection.
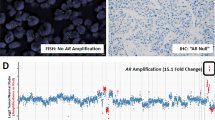
The remaining three cases (cases 20–22) lacked TMPRSS2 rearrangements but showed distinct mutational patterns that are nevertheless supportive of PCa. Case 20 demonstrated focal amplification of the AR gene and deep deletion of PTEN. In case 21, the only pathogenic alteration identified was deep deletion of PTEN. In case 22, the presence of a hotspot TERT promoter mutation would typically provide very strong support for a diagnosis of UCa. However, this case also demonstrated a deep deletion spanning both the MSH6 gene and part of the adjacent MSH2 gene, resulting in microsatellite instability and a very high mutational burden (see Supplemental Fig. 1 and Supplemental Table 2). In addition to the select pathogenic alterations from this case shown in Supplemental Fig. 1 and Supplemental Table 2, there were an additional ~150 mutations identified that are not shown. The findings in this case are concordant with those reported in a prior study of hypermutated PCa, in which loss of MSH2 and/or MSH6 was found to be the most common cause of microsatellite instability in advanced PCa [27]. In contrast, there are no cases of UCa of the bladder with MSH2 and/or MSH6 deep deletion reported in the cBioPortal database. Altogether, the molecular findings in this case are supportive of PCa, and the unexpected presence of a TERT promoter mutation may be a consequence of the high mutational rate.
PCa cases in which molecular analysis was critical for correct diagnosis
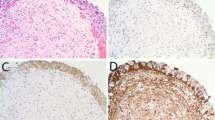
In three cases (15, 16, and 25), molecular analysis was essential for the correct diagnosis of PCa, as the immunoprofile was supportive of UCa. Case 15 demonstrated both squamous and pseudopapillary features (Fig. 3a–d). The GATA3 showed weak staining (+), but the p63 was diffusely strongly positive (+++) and prostate markers were negative, supporting UCa. Case 16 was composed of solid nests with focal pseudopapillary features (Fig. 3e–h). GATA3 was diffusely strongly positive (+++), NKX3.1 showed only rare weakly positive cells (+), and p63 and the remaining prostate markers were negative. Case 25 was composed entirely of solid sheets of cells without glandular or squamous differentiation (Fig. 3i–l). GATA3 and prostate markers were negative, but p63 showed diffusely strongly positive staining (+++). All three of these tumors showed a TMPRSS2-ERG fusion, and case 25 also showed PTEN deletion. No alterations suggestive of UCa were identified. Given the known specificity of the TMPRSS2-ERG fusion for PCa [14, 21], the integrated overall impression for these three cases was revised to PCa based on the molecular findings (Fig. 1, Panel D). Therefore, using integrated molecular analysis, two of these cases (cases 15 and 16), which had originally been diagnosed as UCa, would have been reclassified to PCa; and case 25, originally called HGNOS, would have been further classified as PCa.
Representative hematoxylin and eosin stain, urothelial marker and prostate marker stains for case 15 (a–d), case 16 (e–h) and case 25 (i–l). In all three cases, immunohistochemistry supported UCa and molecular analysis (Fig. 1) supported PCa.
One case that remained ambiguous despite integrated immunohistochemical and molecular analysis
Case 24 was negative for all urothelial and prostatic immunohistochemical markers, and lacked a TERT promoter mutation or TMPRSS2-ERG fusion. Pathogenic alterations in the tumor included truncating mutations in APC and RB1 and hotspot missense mutations in FBXW7, KRAS, and TP53 (Supplemental Fig. 1 and Supplemental Table 2). This mutational profile, and particularly the FBXW7 mutation, favors a diagnosis of UCa, but we do not consider the molecular profile highly specific, and therefore the molecular and integrated overall impressions remain at HGNOS.
Androgen receptor staining
Androgen receptor (AR) had been previously reported to aid in discriminating between poorly differentiated UCa and PCa [28], but the use of AR staining for this purpose has remained controversial in clinical practice. We performed AR staining on all cases in our series and found that immunohistochemistry for AR showed variable positive staining in both UCa cases (9/15 cases, 60%) and PCa cases (6/9 cases, 67%) (Figs. 1 Panel B and 4).
ERG-1 staining
ERG-1 staining was performed to correlate with detection of TMPRSS2-ERG fusions (Fig. 1 Panel B). Of the four cases in which a TMPRSS2-ERG fusion was detected and tissue was available for staining, only one case (case 17) showed diffuse strong staining for ERG-1. The remaining cases with a TMPRSS2-ERG fusion detected by molecular analysis showed either patchy weak staining (cases 15 and 16) or no staining (case 18).
Discussion
Diagnosis of high-grade urothelial versus prostatic carcinoma in the urinary bladder of men previously treated for PCa can be challenging, as the tumor morphology, immunoprofile, and molecular profile can be indeterminate or misleading. Our study of 25 such cases highlights the potentially misleading morphologic, immunohistochemical, and molecular features, which, if given undue weight, may lead to incorrect diagnosis and treatment.
We found that the correct diagnosis of UCa or PCa was supported by immunohistochemistry in the majority of cases, but that integrated molecular analysis was essential for reaching the correct diagnosis in a significant subset of cases. In these three cases that required molecular analysis for the correct diagnosis of PCa, misleading morphologic features included increased nuclear pleomorphism, eosinophilic cytoplasm, squamous differentiation, and pseudopapillary architecture, the latter of which closely mimicked an in situ urothelial component. Squamous differentiation and papillary architecture are very common in UCa, but squamous differentiation is only rarely described in PCa [6]. In addition, while papillary architecture is a known feature in some morphologic variants of PCa, including prostatic ductal and prostatic pseudohyperplastic adenocarcinomas, it has also been reported that pseudopapillary architecture in PCa can closely mimic UCa [5]. The misleading immunohistochemical features in these cases included absence of staining for all prostate markers (with the exception of focal weak NKX3.1 staining in one of the three cases) along with strong and diffuse staining for either p63 or GATA3. Lack of NKX3.1 expression has been reported in prostatic small cell carcinomas [7], but neuroendocrine differentiation was seen in only one of the three NKX3.1-negative prostate cancers (case 25) in our study. Very rare cases of PCa with p63 positivity have been described previously, but unlike our cases, these tumors also maintained expression of prostate specific markers [8, 29]. GATA3-positive PCa have also been rarely described, but unlike our case, the staining was typically in a minority of cells [30]. Therefore, while GATA3 is generally a sensitive and specific marker for differentiating PCa and UCa [3, 31], our study demonstrates that even diffuse and strong GATA3 positivity does not fully exclude a diagnosis of high-grade PCa.
Overall, we found that in contrast to urothelial markers, the prostate markers NKX3.1, PSA, and P501S were entirely specific in this small series of cases, in line with prior studies [32]. Similar to others [6, 7], we also found the sensitivity of these markers in high-grade PCa to be not as good, with several of our PCa cases lacking expression of all three markers. NKX3.1 was the most sensitive of the three prostate markers, showing positive staining in seven of nine PCa cases. With regards to AR, while a prior study found that AR immunohistochemistry was useful in the distinction of high-grade PCa and UCa, with all PCa showing intense diffuse AR positivity and UCa showing only cytoplasmic or weak patchy nuclear staining [28], we found that AR staining was neither sensitive nor specific in our cohort, with variable positive staining seen in both PCa and UCa cases. This very different result might be accounted for at least in part by our post-radiation therapy clinical setting as well as other therapy the patients may have received for PCa. Of note, other investigators have also identified variable AR staining in UCa, which may have implications for future therapeutic approaches [33, 34]. ERG immunohistochemistry has also been recently described as a PCa specific marker, and multiple studies have shown that it can serve as a relatively sensitive and specific marker for TMPRSS2-ERG fusions [35]. In our study cohort, however, we found that ERG immunohistochemical staining was not sensitive as a proxy for ERG fusions, with three out of four cases with TMPRSS2-ERG fusions by molecular analysis showing only weak to negative ERG staining. This poor sensitivity may be the result of disrupted AR signaling and low ERG expression due to the poorly differentiated nature of these tumors and/or the hormone deprivation therapy some of the patients received. Similarly, other studies have shown that ERG immunohistochemistry has poor sensitivity for TMPRSS2-ERG fusions in castration-resistant prostate cancers and prostatic small cell carcinomas [7, 36]. HOXB13 is another prostate-specific immunohistochemical marker shown to be useful in distinguishing PCa from UCa [37], but that marker was not available in our laboratory for testing.
Our study joins multiple others in demonstrating the value of TMPRSS2-ERG fusions and hotspot TERT promoter mutations in distinguishing high-grade PCa from UCa [7, 21, 22]. When one of these alterations is present, it can provide very strong evidence of tumor origin. For that reason, an assay focused on that molecular alteration alone, for example fluorescence in situ hybridization for the TMPRSS2-ERG fusion, could provide a useful and lower cost alternative to the DNA sequencing panel used here. The disadvantage of that more targeted approach is that it will only be informative in cases that harbor TMPRSS2 rearrangements, which are present in only approximately half of PCa cases, whereas a DNA sequencing panel may provide additional molecular clues to the correct diagnosis even in cases lacking TMPRSS2 alterations. In our three PCa cases lacking TMPRSS2 rearrangements, the molecular impression nevertheless provides strong support for the diagnosis of PCa based on alterations, or lack thereof, in other genes. Similarly, PCR-based TERT promoter testing, while very useful and also less expensive than a targeted sequencing panel, may in rare instances provide a misleading result in high-grade PCa. One of the most striking of these cases in our study was case 22, a PCa case lacking a TMPRSS2 rearrangement which also demonstrated a hotspot TERT promoter mutation. If taken in isolation, this hotspot TERT promoter mutation would have provided strong support for a diagnosis of UCa. However, the larger molecular context (deletion of MSH6 and part of MSH2 with resulting microsatellite instability) instead supports the correct diagnosis of PCa, in agreement with the immunohistochemical features of the case. Therefore, our study also demonstrates the added value of a more comprehensive DNA sequencing panel, which provides additional patterns of molecular alterations to suggest prostatic or urothelial origin, in a subset of cases.
We acknowledge that our study is limited by case selection, and a precise sensitivity and specificity that can be applied broadly when utilizing these ancillary studies cannot be determined, as these would depend on level of suspicion for an ambiguous case and pathologist experience. With regards to the validity of our molecular testing, we used a clinically validated and implemented DNA sequencing assay. While the advantage of this approach is detection of a wide range of alterations that can inform both diagnosis and treatment, one disadvantage is that a small subset of gene fusions will not be detected. Our sequencing assay covers all of the exons for both TMPRSS2 and ERG, as well as introns 1 and 2 of TMPRSS2. Based on the spatial distribution of TMPRSS2 fusion breakpoints [38], we expect to detect more than 90% of TMPRSS2 fusions with ERG or other ETS family genes. However, the small subset of fusions with breakpoints in other introns of the TMPRSS2 gene are likely to be missed. In addition, in a small number of prostate cancers, ERG is fused to a gene other than TMPRSS2, most commonly SLC45A3 [39], and our sequencing assay would most likely miss those fusions given the absence of ERG intronic coverage. One approach that may improve fusion detection sensitivity in targeted sequencing assays is the addition of RNA sequencing for fusion transcripts, which may eliminate the need for broad intronic coverage. However, the sensitivity of RNA fusion analysis in our cohort might be limited by disrupted AR signaling and low fusion transcript expression.
Taken together, the findings in the literature and in the present study suggest several practical considerations for diagnosis of bladder masses in patients previously treated for prostate cancer. First, in most of these cases, the morphologic features will be sufficient for diagnosis. Such cases were excluded from the present study, as ancillary studies would be unnecessary. When the carcinoma in the bladder is high-grade/poorly differentiated, it is important to recognize morphology that is ambiguous for PCa versus UCa and to consider additional workup. Second, any individual immunohistochemical or molecular finding may be unreliable or misleading in the high-grade/poorly differentiated setting, and use of multiple molecular and immunohistochemical findings simultaneously can provide a more comprehensive diagnostic impression and avoid misdiagnosis. Third, while our study primarily focuses on the diagnostic utility of molecular analysis, our results also highlight the added value of a DNA sequencing panel in identifying specific molecular alterations that may be targetable. Patients with microsatellite unstable PCa (such as case 22) are eligible for and more likely to benefit from immunotherapy [40]. Other genes in which potentially targetable alterations were identified in our study include PIK3CA (cases 7, 14, and 18), FGFR3 (case 11), BRCA2 (case 20), BRIP1 (case 23), MRE11A (case 19), and TSC1 (cases 9 and 14). Thus, targeted DNA sequencing may provide both diagnostic and therapeutic value, and the decision to use this more expensive and comprehensive test should be considered by both the pathologist and/or treating physician based on the pathologic findings and larger clinical context. Although next generation sequencing is not readily available in most community practices, clinically validated tests are becoming more widely available through a variety of both academic centers and private companies.
References
Chuang A-Y, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. Am J Surg Pathol. 2007;31:1246–55.
Epstein JI, Egevad L, Humphrey PA, Montironi R, Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the prostate: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38:e6–19.
Chang A, Amin A, Gabrielson E, Illei P, Roden RB, Sharma R, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472–6.
Amin MB, Trpkov K, Lopez-Beltran A, Grignon D, Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the bladder lesions: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38:e20–34.
Gordetsky J, Epstein JI. Pseudopapillary features in prostatic adenocarcinoma mimicking urothelial carcinoma: a diagnostic pitfall. Am J Surg Pathol. 2014;38:941–5.
Parwani AV, Kronz JD, Genega EM, Gaudin P, Chang S, Epstein JI. Prostate carcinoma with squamous differentiation: an analysis of 33 cases. Am J Surg Pathol. 2004;28:651–7.
Lotan TL, Gupta NS, Wang W, Toubaji A, Haffner MC, Chaux A, et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod Pathol. 2011;24:820–8.
Osunkoya AO, Hansel DE, Sun X, Netto GJ, Epstein JI. Aberrant diffuse expression of p63 in adenocarcinoma of the prostate on needle biopsy and radical prostatectomy: report of 21 cases. Am J Surg Pathol. 2008;32:461–7.
Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846–50.
Wheeler JA, Zagars GK, Ayala AG. Dedifferentiation of locally recurrent prostate cancer after radiation therapy. Evidence for tumor progression. Cancer. 1993;71:3783–7.
Siders DB, Lee F. Histologic changes of irradiated prostatic carcinoma diagnosed by transrectal ultrasound. Hum Pathol. 1992;23:344–51.
Mazzone E, Mistretta FA, Knipper S, Palumbo C, Tian Z, Pecoraro A, et al. Long-term incidence of secondary bladder and rectal cancer in patients treated with brachytherapy for localized prostate cancer: a large-scale population-based analysis. BJU Int. 2019. https://doi.org/10.1111/bju.14841.
Moschini M, Zaffuto E, Karakiewicz PI, Andrea DD, Foerster B, Abufaraj M, et al. External beam radiotherapy increases the risk of bladder cancer when compared with radical prostatectomy in patients affected by prostate cancer: a population-based analysis. Eur Urol. 2019;75:319–28.
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–25.
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22.
Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171:540–5.e25.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Disco. 2012;2:401–4.
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6.
Stoehr R, Taubert H, Zinnall U, Giedl J, Gaisa NT, Burger M, et al. Frequency of TERT promoter mutations in prostate cancer. Pathobiology. 2015;82:53–7.
Lara PN, Heilmann AM, Elvin JA, Parikh M, de Vere White R, Gandour-Edwards R, et al. TMPRSS2-ERG fusions unexpectedly identified in men initially diagnosed with nonprostatic malignancies. JCO Precis Oncol. 2017;2017. https://doi.org/10.1200/PO.17.00065.
Alaghehbandan R, Vanecek T, Trpkov K, Comperat E, Kristiansen G, Svajdler M, et al. High-grade adenocarcinoma of the prostate mimicking urothelial carcinoma is negative for TERT mutations. Appl Immunohistochem Mol Morphol. 2019;27:523–8.
Williamson SR, Zhang S, Yao JL, Huang J, Lopez-Beltran A, Shen S, et al. ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: evidence supporting monoclonal origin. Mod Pathol. 2011;24:1120–7.
Mannan R, Taylor AS, Spratt DE, Chinnaiyan AM, Montgomery JS, Brown NA, et al. TERT- beyond the territory: Usage of PCR-based TERT promoter assay in defining urothelial carcinoma in a case of long-standing prostatic adenocarcinoma. Pathol Res Pract. 2019;152663.
Joseph NM, Tsokos CG, Umetsu SE, Shain AH, Kelley RK, Onodera C, et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol. 2019;248:164–78.
Rausch T, Zichner T, Schlattl A, Stütz AM, Benes V, Korbel JO. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics. 2012;28:i333–9.
Pritchard CC, Morrissey C, Kumar A, Zhang X, Smith C, Coleman I, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988.
Downes MR, Torlakovic EE, Aldaoud N, Zlotta AR, Evans AJ, van der Kwast TH. Diagnostic utility of androgen receptor expression in discriminating poorly differentiated urothelial and prostate carcinoma. J Clin Pathol. 2013;66:779–86.
Tan H-L, Haffner MC, Esopi DM, Vaghasia AM, Giannico GA, Ross HM, et al. Prostate adenocarcinomas aberrantly expressing p63 are molecularly distinct from usual-type prostatic adenocarcinomas. Mod Pathol. 2015;28:446–56.
Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38:13–22.
Mohanty SK, Smith SC, Chang E, Luthringer DJ, Gown AM, Aron M, et al. Evaluation of contemporary prostate and urothelial lineage biomarkers in a consecutive cohort of poorly differentiated bladder neck carcinomas. Am J Clin Pathol. 2014;142:173–83.
Gurel B, Ali TZ, Montgomery EA, Begum S, Hicks J, Goggins M, et al. NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol. 2010;34:1097–105.
Tyagi A, Chandrasekaran B, Kolluru V, Rai S, Jordan AC, Houda A, et al. Combination of androgen receptor inhibitor and cisplatin, an effective treatment strategy for urothelial carcinoma of the bladder. Urol Oncol. 2019;37:492–502.
Necchi A, Lo Vullo S, Giannatempo P, Raggi D, Perrone F, Nicolai N, et al. Association of androgen receptor expression on tumor cells and PD-L1 expression in muscle-invasive and metastatic urothelial carcinoma: insights for clinical research. Clin Genitourin Cancer. 2018;16:e403–10.
Shah RB. Clinical applications of novel ERG immunohistochemistry in prostate cancer diagnosis and management. Adv Anat Pathol. 2013;20:117–24.
Udager AM, Shi Y, Tomlins SA, Alva A, Siddiqui J, Cao X, et al. Frequent discordance between ERG gene rearrangement and ERG protein expression in a rapid autopsy cohort of patients with lethal, metastatic, castration-resistant prostate cancer. Prostate. 2014;74:1199–208.
Varinot J, Cussenot O, Roupret M, Conort P, Bitker M-O, Chartier-Kastler E, et al. HOXB13 is a sensitive and specific marker of prostate cells, useful in distinguishing between carcinomas of prostatic and urothelial origin. Virchows Arch. 2013;463:803–9.
Krumbholz M, Agaimy A, Stoehr R, Burger M, Wach S, Taubert H, et al. Molecular composition of genomic TMPRSS2-ERG rearrangements in prostate cancer. Dis Markers. 2019;2019:5085373.
Esgueva R, Perner S, LaFargue CJ, Scheble V, Stephan C, Lein M, et al. Prevalence of TMPRSS2–ERG and SLC45A3–ERG gene fusions in a large prostatectomy cohort. Mod Pathol. 2010;23:539–46.
Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–8.
Acknowledgements
This study was funded by the UCSF Department of Pathology Clinical Research Endowment awards granted to EC, KG, and BS. Capture-based next generation sequencing was performed at the UCSF Clinical Cancer Genomics Laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chan, E., Garg, K. & Stohr, B.A. Integrated immunohistochemical and molecular analysis improves diagnosis of high-grade carcinoma in the urinary bladder of patients with prior radiation therapy for prostate cancer. Mod Pathol 33, 1802–1810 (2020). https://doi.org/10.1038/s41379-020-0543-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0543-y
This article is cited by
-
p53 null phenotype is a “positive result” in urothelial carcinoma in situ
Modern Pathology (2022)