Abstract
Microscopic satellite metastases are an adverse prognostic feature in primary cutaneous melanoma patients. The prognostic significance of microsatellites, including their number, size and distance from the primary melanoma, using the 8th edition American Joint Committee on Cancer definition, has not previously been evaluated. This study sought to determine the prognostic significance of microsatellites in histopathologically reviewed cases. Eighty-seven cases of primary cutaneous melanoma with the presence of microsatellites documented in the original pathology report and all histopathology slides available were reviewed and the findings were correlated with clinical outcome. Matched control cases were selected for all confirmed microsatellites cases. The presence of microsatellites was confirmed in 69 cases. The microsatellite group had significantly worse prognosis, with 21% 5-year disease-free survival compared with 56% in the control group (p < 0.001). The 5-year melanoma-specific survival was 53% in the microsatellites group and 73% in the control group (p = 0.004). Increasing distance (mm) of the microsatellite from the primary melanoma was found to adversely influence disease-free survival (HR = 1.24, 95% CI: 1.13–1.36, p < 0.001), overall survival (HR = 1.26 95%CI: 1.13–1.40, p < 0.001), and melanoma-specific survival (HR = 1.27 95% CI: 1.11–1.45, p < 0.001). Number and size of microsatellites were not significant prognostic factors. The presence of microsatellites was the only factor that proved to be an independent predictor of sentinel node positivity in multivariate analysis (OR 4.64; 95% CI 1.66–12.95; p = 0.003). Microsatellites were significantly associated with more loco-regional recurrences (p < 0.001) but not distant metastases (p = 0.821). Melanomas with microsatellites as defined by the 8th edition American Joint Committee on Cancer staging system are thus aggressive tumors, associated with significantly worse disease-free survival, overall survival and melanoma-specific survival. The presence of microsatellites is also associated with sentinel node-positivity and local and in-transit recurrence. Increasing distance of the microsatellite from the primary tumor is an independent adverse prognostic factor that warrants further evaluation.
Similar content being viewed by others
Introduction
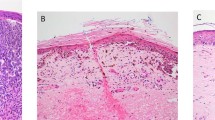
Microsatellite, satellite and intransit melanoma metastases are types of non-nodal locoregional metastases that are thought to occur as a consequence of intralymphatic or possibly angiotropic tumor spread [1]. They are grouped together, regardless of the number of lesions present, as components of the N category in the 8th edition of the American Joint Committee on Cancer (AJCC) melanoma staging system [1, 2]. Microsatellites are microscopic cutaneous and/or subcutaneous metastases found adjacent to or deep to a primary melanoma on pathological examination of the primary tumor site, usually in wide excision specimens (Figs. 1 and 2). In the previous (7th) edition of the AJCC melanoma staging system, minimum size and distance thresholds were utilized; microsatellites were defined as ‘discontinuous nests of metastatic cells more than 0.05 mm in diameter that are clearly separated by normal dermis (not fibrosis or inflammation) from the main invasive component of melanoma by a distance of at least 0.3 mm [3]. In the 8th edition of the AJCC melanoma staging system, the definition of a microsatellite was clarified and refined. It is now defined as “a microscopic cutaneous and/or subcutaneous metastasis adjacent to or deep to and completely discontinuous from a primary melanoma with unaffected stroma occupying the space between, identified on pathological examination of the primary tumor site”. Satellites are defined empirically as any foci of clinically evident cutaneous and/or subcutaneous metastases occurring within 2 cm of but discontinuous from the primary melanoma and in-transit metastases are similarly defined but occurring >2 cm from the primary melanoma in the region between the primary and the regional lymph node basin. The presence of microsatellites in the primary melanoma tumor specimen (or satellite or intransit metastases) upstages patients to stage III. This categorization is based on studies that show that the presence of microsatellites (and satellite and in transit metastases) portends a relatively poor prognosis, including increased frequency of positive sentinel nodes, an increased risk of loco-regional recurrence [4,5,6,7,8,9,10,11] and worse disease-free survival [8,9,10, 12,13,14] and overall survival [4,5,6,7,8, 11, 12] When the 8th edition AJCC database was analyzed, there were no significant differences in survival outcome for patients with microsatellite, satellite and in transit metastases and hence they were grouped together for staging purposes.
The primary aim of this study was to determine the prognostic value of microsatellites in patients with primary cutaneous melanomas managed in the contemporary sentinel node biopsy era, utilizing the AJCC 8th edition definition of microsatellites in histopathologically reviewed cases. Secondary aims were to analyze the associations of microsatellites with sentinel positivity and type of first recurrence, and to assess the influence on survival of the number of microsatellites, their size and their distance from the primary tumor.
Methods
Patients with primary invasive cutaneous melanomas treated at the Melanoma Institute Australia between 2001 and 2011 for whom the presence of microsatellites was recorded and all histopathology slides were available for review (n = 87), were selected from the Melanoma Institute Australia database. Parameters recorded for each case were: age, gender, date of primary diagnosis, date and type of recurrence, date of last follow-up, status at last follow-up date, stage (AJCC 8th edition), date and status of therapeutic lymph node dissection or sentinel node biopsy and completion lymph node dissection, primary tumor thickness, ulcerative status, tumor mitotic rate (number of mitoses per mm²), lymphovascular invasion, regression (early [presence of tumor-infiltrating lymphocytes], intermediate and late), melanoma subtype and predominant cell type. A microsatellite was defined, as in the 8th edition AJCC staging system, as “a microscopic cutaneous and/or subcutaneous metastasis adjacent to or deep to and completely discontinuous from a primary melanoma with unaffected stroma occupying the space between, identified on pathological examination of the primary tumor site. Fibrous scarring and/or inflammation noted between an apparently separate nodule and the primary tumor (rather than normal stroma) may represent regression of the intervening tumor; if these findings are present, the nodule is considered to be an extension of the primary tumor and not a microsatellite.” Tumor-infiltrating lymphocytes are defined as “lymphocytes in direct apposition with tumor cells”. Intermediate regression is defined as the presence of early angiofibroplasia (with morphological characteristics of an immature scar). Late regression is defined as mature fibrosis often accompanied by loss of rete ridges in the overlying epidermis [15].
Histopathologic specimens of the 87 cases were collected and reviewed by a research fellow (MGN) and by specialist melanoma pathologists from the Department of Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital, Sydney (RZK and RAS). After pathologic review, 18 cases were excluded because the initial diagnosis of microsatellites recorded in the Melanoma Institute Australia database was not confirmed on review of the pathology slides. In each instance, these cases were excluded because, whilst the tumor deposits may have appeared separate from the primary melanoma and fulfilled the size and distance requirements for the 7th edition AJCC staging system definition of microsatellites, on review it was apparent or appeared likely that the “microsatellites” were connected to the primary tumor (or only separated by reactive fibrous tissue) and hence did not fulfill the AJCC 8th edition definition of microsatellites. For each confirmed microsatellite case, the number of microsatellites, size of the largest microsatellite (mm), i.e., greatest diameter, and greatest distance from the primary tumor (mm) was recorded. Where the primary tumor or previous scar related to prior excision was not present in the same section of the microsatellite, the distance of the microsatellite from the primary tumor was calculated using an assumed thickness of 3 mm per tissue block. These three parameters were analyzed both as continuous variables and as categorical variables, with cut-off points based on the median, and 25th and 75th percentiles. The 69 microsatellites cases were matched with 69 control cases without microsatellites for age, gender, year of diagnosis and T stage (AJCC 8th edition). None of the patients included in this study had clinically apparent satellite or in-transit metastases at the time of the melanoma resection.
Local recurrence was defined as recurrence of melanoma within 5 cm of the wide excision scar in cases in which the primary tumor was completely excised with histological clear margins. In-transit recurrence was defined as recurrence occurring outside a 5 cm radius from the primary melanoma or scar, between the primary site and the regional node field. For the purposes of analysis, local, in-transit and regional node recurrences were grouped together as loco-regional recurrences.
For analysis, the primary melanoma subtypes were grouped into four categories: (1) superficial spreading melanoma, (2) nodular melanoma, (3) desmoplastic melanoma and (4) other (acral lentiginous, lentigo maligna melanoma and blue nevus-like melanoma).
Disease-free survival and melanoma-specific survival were calculated from the date of primary melanoma diagnosis to the date of first recurrence or death from melanoma, respectively.
All statistical analyses were performed using IBM SPSS Statistic version 21.0 software (Chicago, IL) and R version 3.4.4 (R Core Team, Vienna, Austria). Survival curves were summarized using the Kaplan–Meier method and differences between curves were assessed using the log-rank test. An adjusted Cox proportional hazards model was run to confirm the association between microsatellites and survival outcomes after accounting for other prognostic factors. Variables with a p value < 15% were included in the multivariable regression model. Log minus log plots were visually inspected to assess the proportional hazards assumption. Mann–Whitney U, chi-square, and multivariable binary logistic regression methods were used to assess the associations between features of microsatellites, sentinel nodepositivity and nonsentinel nodepositivity.
A sensitivity analysis was performed to compare the survival outcomes of the 18 cases excluded on pathological review with the analyzed cohorts.
Results
The study cohort consisted of 69 patients who had primary cutaneous melanomas with microsatellites and 69 matched control melanoma patients without microsatellites. Clinical and histopathologic characteristics of the study population are presented in Table 1. Median follow-up was 75 months (95% CI 70–94 months) in the microsatellites group and 80 months (95% CI 64–110 months) in the control group. In the microsatellites group the median number of microsatellites was 1 (range 1–10), the median size of the microsatellites was 1.20 mm (range 0.09–16.00 mm), and the median distance from the primary tumor to the microsatellites was 2.20 mm (range 0.10–30.00 mm; Table 2 and Fig. 3).
Factors associated with microsatellites
The presence of lymphovascular invasion was significantly associated with the presence of microsatellites (20 and 4 cases in the microsatellites and control groups, respectively, p < 0.001). A predominantly epithelioid cell type was observed in 73% of microsatellites cases compared with 42% of control cases (p < 0.001). Superficial spreading melanomas were associated with the presence of microsatellites, and desmoplastic melanomas were more frequently observed in the control group (p = 0.020). The microsatellites group had a lower proportion of associated nevi in the microsatellites group compared with the control group, but this did not reach statistical significance (6% versus 20%; p = 0.053).
Prognostic features of microsatellites
A sub-group analysis of the 69 microsatellites cases was performed to determine the prognostic influence of the number, size, and distance of the microsatellites from the primary tumor. The number of microsatellites did not influence disease-free survival, overall survival or melanoma-specific survival in univariable analysis (corresponding HR (95% CI) and p value were 0.96 (0.81–1.13) p = 0.606, 0.96 (0.83–1.10) p = 0.528 and 0.94 (0.83–1.352) p = 0.352, respectively; Table 3).
The distance of the microsatellites from the primary melanoma as a continuous variable was found to significantly influence disease-free survival, overall survival and melanoma-specific survival on univariable analysis (p < 0.001 for all three outcomes; Table 3). These differences retained significance in the multivariable model (HR 1.18; 95% CI 1.06–1.32; p = 0.003, HR 1.22; 95% CI 1.07–1.38 and HR 1.30; 95% CI 1.12–1.51; p < 0.001, respectively). When distance from the primary tumor was analyzed as a categorical variable, microsatellites distance 2.21–3.75 mm and >3.75 mm distant had a significantly worse prognosis compared with microsatellites 0.01–1.00 mm distant, in terms of disease-free survival (p = 0.006 and p = 0.003, respectively). Moreover, distance categories 1.01–2.20 mm, 2.21–3.75 mm and >3.75 mm were all associated with reduced overall survival compared with microsatellites 0.01–1.00 mm distant (p = 0.123, p = 0.064 and p = 0.001, respectively; Table 3). The sensitivity analysis showed the survival curves of the 18 cases excluded on pathological review fell in between the confirmed microsatellites cases and the control ones for disease-free survival, overall survival, melanoma-specific survival, loco-regional disease-free survival and distant disease-free survival (Supplementary Fig. 1A–E).
The microsatellite size as a continuous variable significantly influenced melanoma-specific survival on univariable analysis (p < 0.001). This difference remains on multivariable analysis (p < 0.001). On univariable analysis, the size of the microsatellites as a continuous variable influenced disease-free survival (p < 0.001). However, this difference lost significance on multivariable analysis (p = 0.066). When the microsatellites group was divided into two categories, based on the microsatellite size with a cut-off point at 1.20 mm, the >1.20 mm group had significantly reduced disease-free survival on univariable analysis (p = 0.002), although this significant difference was lost in the multivariable model (p = 0.107). No significant differences in melanoma-specific survival were observed between the two categories on both univariable (p = 0.552; Table 3) and multivariable analysis (p = 0.125).
Recurrence and survival in the overall cohort
Table 4 displays the univariable analysis of survival outcomes and potential predictors using the overall cohort. Recurrence during the follow-up period occurred in a total of 68 patients in the overall cohort. In the microsatellites group 57% (n = 39) developed a recurrence compared with 42% (n = 29) in the control group (p < 0.001). Specifically, 54% and 38% of microsatellite cases experienced a loco-regional or distant recurrence respectively, compared with 26% and 31% of cases without microsatellites (p < 0.001 and p = 0.821). The microsatellites group had a significantly worse disease-free survival, with 23% (95% CI 14–37%) and 21% (95% CI 12–35%) cumulative disease-free survival at 3 and 5 years, respectively, compared with 70% (95% CI 59–82%) and 56% (95% CI 45–70%) in the control group (p < 0.001) (Fig. 4c). This difference was still significant after adjusting for potential confounders (adjusted HR 3.14; 95% CI 1.88–5.24; p < 0.001). Three and 5-year cumulative melanoma-specific survival was 68% (95% CI 57–82%) and 53% (95% CI 41–70%) in the microsatellites group and 86% (95% CI 78–95%) and 73% (95% CI 63–85) in the control group, respectively (p = 0.004) (Fig. 4b). This difference still remains significant in the adjusted Cox regression (HR 2.21; 95% CI 1.18–4.11; p = 0.012). For overall survival, 3 and 5-year overall survival was 57% (95% CI 45.1–71%) and 43% (95% CI 31–58%) in the microsatellites group and 86% (95% CI 78–95%) and 73.1% (95% CI 63–85) in the control group, respectively (p < 0.001) (Fig. 4a). This difference was also consistent with the adjusted Cox regression (HR 2.60; 95% CI 1.55–4.37; p < 0.001).
Sentinel and nonsentinel lymph node positivity
Sentinel node biopsy was conducted in 41 microsatellites patients and 42 controls. On univariable analysis, the presence of microsatellites (p = 0.003), lymphovascular invasion (p = 0.039) and epithelioid cell type (p = 0.027) were associated with a positive sentinel node biopsy. The presence of microsatellites was the only factor that proved to be an independent predictor of sentinel node positivity after adjustment for other variables in the multivariable model (OR 4.64; 95% CI 1.66–12.95; p = 0.003). No significant predictors of nonsentinel node positivity were identified on either univariable or multivariable analysis (n = 24).
Discussion
This study confirms that the presence of microsatellites, as defined in the 8th edition AJCC melanoma staging system, negatively influences melanoma-specific survival and disease-free survival. Furthermore, microsatellites increased the likelihood of sentinel node positivity and loco-regional recurrence. The findings also indicate that an increased distance of the microsatellite from the primary tumor conveys a worse prognosis, both in terms of melanoma-specific survival and disease-free survival. However, neither the size nor the number of microsatellites present in the primary tumor specimen was prognostic.
Several previous studies have reported that the presence of microsatellites is a predictor of reduced overall survival and disease-free survival [5, 8,9,10, 12, 14]. The 5-year cumulative overall survival ranged from 34% to 40% in the majority of these studies, and 5-year cumulative disease-free survival ranged from 18% to 36% [5, 10, 12, 14]. These results are comparable with the results for cases with microsatellites in the present study (5-year overall survival: 43, 5-year melanoma-specific survival: 53% and 5-year disease-free survival: 21%).
Of the previous studies only one, reported by Leon et al. in 1991, was a case–control study [14]. Moreover, different definitions have been used in various studies to describe microsatellites. Nagore et al. reported overall survival and disease-free survival rates of 70% and 52%, respectively for patients with microsatellites, although survival was still significantly worse for microsatellites cases compared with the cohort of patients without microsatellites [8]. However, this study analyzed a melanoma patient cohort with nonaggressive pathologic features (the median Breslow thickness was only 1.2 mm and ulceration was present in only 21% of the melanomas). In comparison, other studies have shown that melanomas with microsatellites were associated with aggressive pathologic features. For example, in a study conducted by Kimsey et al., median Breslow thickness was 5.4 mm and 71% of the melanomas were ulcerated [5]. In our study, median Breslow thickness was 4.1 mm and 62% of the melanomas were ulcerated. This emphasizes the importance of matching microsatellites cases and controls for Breslow thickness and ulceration.
Lymphovascular invasion has been shown to be an important prognostic factor in breast cancer [16]. In cutaneous melanoma, however, the prognostic significance of lymphovascular invasion remains unclear, as several studies have failed to show any prognostic value of this pathologic feature [17,18,19,20,21,22]. Our study results showed that the presence of microsatellites was associated with lymphovascular invasion, and lymphovascular invasion was a prognostic factor for worse melanoma-specific survival and disease-free survival in the univariable analysis. Lymphovascular invasion was also correlated with sentinel node positivity. The association of lymphovascular invasion and microsatellites could be explained by postulating that the presence of microsatellites is the result of lymphovascular transport of tumor cells to location in the vicinity of the primary tumor. Furthermore, it seems logical that the further from the primary tumor the microsatellites are located, the greater their impact on melanoma-specific survival and disease-free survival. This theory is supported by our study results, as increased distance between microsatellites and the primary tumor was associated with reduced melanoma-specific survival and disease-free survival. A possible alternative explanation for why microsatellites located closer to the primary tumor are associated with a more favorable prognosis is that some (a minority of) putative microsatellites closer to the primary are not true microsatellites at all (i.e., they represent unrecognized direct extension of the primary tumor). In contrast, microsatellites located further away from the primary tumor are probably all true microsatellites.
Recurrences occurred in 51% of patients in the microsatellites group, compared with 30% in the control group (p = 0.015). This is in accordance with other studies that analyzed recurrences in microsatellites cases [4, 5, 9, 10]. The majority of first recurrences in patients who had been found to have microsatellites were local or in-transit. This observation could be explained by the hypothesis that these local recurrences are actually local metastases that had already spread so far from the primary tumor that they were not excised at the time of wide excision of the primary tumor. Further studies investigating the prognostic and biological significance of microsatellites in larger, ideally multi-institutional cohorts are needed to overcome the challenge of fully understanding an infrequently observed feature. The incidence of microsatellites in primary cutaneous melanomas is 4–19% in the literature [5, 9, 12,13,14]. However, in the Melanoma Institute Australia database, the presence of microsatellites was reported in only 3% of 4313 pathology reports.
A diagnosis of microsatellites may not always be correct i.e., what is called a microsatellite in the pathology report may not actually represent a true microsatellite (i.e., local metastasis or even a focus of angiotropic spread). This is due to the fact that the presence of an isolated nest of tumor cells adjacent to a primary tumor can be a direct extension of the primary tumor that has been cut tangentially or in cross-section. The nest of tumor cells may appear separate from the primary tumor in the plane of sectioning examined but may in fact be joined to it in a different plane (Fig. 5a, b).
a A dermal nest of invasive melanoma appears to be separated from the overlying adjacent invasive tumor in this plane of section and could be erroneously interpreted as a microsatellite if additional sections were not examined. b Deeper sections reveal that the two apparently separate melanoma nodules in a are connected (and represent periadnexal extension of melanoma). When there is uncertainty regarding the presence of a microsatellite, examination of additional sections may clarify this issue.
Therefore, we recommend that serial sectioning should be performed on the tissue blocks to prevent a false-positive diagnosis of microsatellites, as occurred in our series. If microsatellites are found a significant distance away from the primary tumor then serial sectioning would not be required. However, if it is not possible to be certain based on morphological examination whether the possible microsatellite could represent direct continuity of the main tumor in another plane of section not visible in the initial sections, it would prudent to cut additional sections to attempt to clarify this.
In conclusion, the presence of microsatellites is associated with the presence of positive sentinel nodes, an increased likelihood of loco-regional recurrence and reduced disease-free survival and melanoma-specific survival. Besides the presence or absence of microsatellites, the greatest distance of the microsatellites from the primary tumor is also an important prognostic factor. There is no doubt that identification of microsatellites will continue to be a factor that influences the staging and clinical management of melanoma patients.
References
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–92.
Gershenwald JE, Scolyer RA, Hess KR, Thompson JF, Long GV, Ross MI, et al. Melanoma of the skin, In: Amin MB, Edge SB, editors. AJCC cancer staging manual. 8th ed. New York: Springer; 2017. p. 563–85.
Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206.
Kelly JW, Sagebiel RW, Calderon W, Murillo L, Dakin RL, Blois MS. The frequency of local recurrence and microsatellites as a guide to reexcision margins for cutaneous malignant melanoma. Ann Surg. 1984;200:759–63.
Kimsey TF, Cohen T, Patel A, Busam KJ, Brady MS. Microscopic satellitosis in patients with primary cutaneous melanoma: implications for nodal basin staging. Ann Surg Oncol. 2009;16:1176–83.
Mocellin S, Ambrosi A, Montesco MC, Foletto M, Zavagno G, Nitti D, et al. Support vector machine learning model for the prediction of sentinel node status in patients with cutaneous melanoma. Ann Surg Oncol. 2006;13:1113–22.
Mraz-Gernhard S, Sagebiel RW, Kashani-Sabet M, Miller JR 3rd, Leong SP. Prediction of sentinel lymph node micrometastasis by histological features in primary cutaneous malignant melanoma. Arch Dermatol. 1998;134:983–7.
Nagore E, Oliver V, Botella-Estrada R, Moreno-Picot S, Insa A, Fortea JM. Prognostic factors in localized invasive cutaneous melanoma: high value of mitotic rate, vascular invasion and microscopic satellitosis. Melanoma Res. 2005;15:169–77.
Rao UN, Ibrahim J, Flaherty LE, Richards J, Kirkwood JM. Implications of microscopic satellites of the primary and extracapsular lymph node spread in patients with high-risk melanoma: pathologic corollary of Eastern Cooperative Oncology Group Trial E1690. J Clin Oncol. 2002;20:2053–7.
Shaikh L, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR 3rd, Kashani-Sabet M. The role of microsatellites as a prognostic factor in primary malignant melanoma. Arch Dermatol. 2005;141:739–42.
Tejera-Vaquerizo A, Solis-Garcia E, Rios-Martin JJ, Moreno-Ramirez D. [Primary cutaneous melanoma: prognostic factors not included in the classification of the American Joint Committee on Cancer]. Actas Dermosifiliogr. 2011;102:255–63.
Day CL Jr., Harrist TJ, Gorstein F, Sober AJ, Lew RA, Friedman RJ, et al. Malignant melanoma. Prognostic significance of “microscopic satellites” in the reticular dermis and subcutaneous fat. Ann Surg. 1981;194:108–12.
Harrist TJ, Rigel DS, Day CL Jr., Sober AJ, Lew RA, Rhodes AR, et al. “Microscopic satellites” are more highly associated with regional lymph node metastases than is primary melanoma thickness. Cancer. 1984;53:2183–7.
Leon P, Daly JM, Synnestvedt M, Schultz DJ, Elder DE, Clark WH Jr. The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg. 1991;126:1461–8.
Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678–83.
Mohammed RA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol. 2007;31:1825–33.
Barnhill RL, Fine JA, Roush GC, Berwick M. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer. 1996;78:427–32.
Drzewiecki KT, Frydman H, Andersen K, Poulsen H, Ladefoged C, Vibe P. Malignant melanoma. Changing trends in factors influencing metastasis-free survival from 1964 to 1982. Cancer. 1990;65:362–6.
Petitt M, Allison A, Shimoni T, Uchida T, Raimer S, Kelly B. Lymphatic invasion detected by D2-40/S-100 dual immunohistochemistry does not predict sentinel lymph node status in melanoma. J Am Acad Dermatol. 2009;61:819–28.
Sahni D, Robson A, Orchard G, Szydlo R, Evans AV, Russell-Jones R. The use of LYVE-1 antibody for detecting lymphatic involvement in patients with malignant melanoma of known sentinel node status. J Clin Pathol. 2005;58:715–21.
Vollmer RT. Malignant melanoma. A multivariate analysis of prognostic factors. Pathol Annu. 1989;24(Pt 1):383–407.
Worth AJ, Gallagher RP, Elwood JM, Yang PC, Lamb C, Spinelli JJ, et al. Pathologic prognostic factors for cutaneous malignant melanoma: the Western Canada Melanoma Study. Int J Cancer. 1989;43:370–5.
Acknowledgements
Support from colleagues at Melanoma Institute Australia and the Royal Prince Alfred Hospital is gratefully acknowledged. RAS is supported by the Australian National Health and Medical Research Council Fellowship program. JFT is supported by the Melanoma Foundation of the University of Sydney. RVR is supported by a clinical researcher scholarship from Sydney Research. This research was also supported by research program grants from Cancer Institute New South Wales and the Australian National Health and Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JFT has received honoraria for advisory board participation from BMS Australia, MSD Australia, GSK and Provectus Inc, and travel support from GSK and Provectus Inc. RAS has received fees for professional services from Merck Sharp & Dohme, GlaxoSmithKline Australia, Bristol-Myers Squibb, Dermpedia, Novartis Pharmaceuticals Australia Pty Ltd, Myriad, NeraCare and Amgen.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Niebling, M.G., Haydu, L.E., Lo, S.N. et al. The prognostic significance of microsatellites in cutaneous melanoma. Mod Pathol 33, 1369–1379 (2020). https://doi.org/10.1038/s41379-020-0500-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0500-9
This article is cited by
-
Histological regression in melanoma: impact on sentinel lymph node status and survival
Modern Pathology (2021)