Abstract
Immune checkpoint inhibitors (ICI) can induce a durable response against a wide range of malignancies but cause immune related adverse events. The purpose of this study was to evaluate whether the pattern of inflammation in a liver biopsy in patients on ICIs is likely to be related to ICIs or other causes, and whether the pattern correlates with LFT abnormalities, imaging findings, and responsiveness to steroids. Cancer patients on ICIs who underwent liver biopsy were identified. Clinical data were obtained from electronic records. Liver biopsies were recorded as hepatitic, cholangitic, mixed, steatotic, or as mild nonspecific changes. In total, 28 liver biopsies had a predominantly hepatitic pattern of inflammation, including 11 biopsies with granulomas and 10 with endothelialitis. Eight biopsies had a mixed hepatocytic and cholangitic pattern of injury, including 6 with granulomas and 4 with endothelialitis. Sixteen patients had a predominantly cholangitic pattern, with portal-based inflammation. Three patients had a pattern resembling fatty liver, and five had mild nonspecific changes. The three most common histologic patterns correlated with the pattern of LFT abnormalities. The majority of patients with a cholangitic pattern had competing causes for elevated LFTs, including disease progression or concomitant chemotherapy. The cholangitic pattern was more likely to have bile duct dilatation or narrowing on liver imaging. The pattern of inflammation, degree of lobular injury, or presence of granulomas or endothelialitis did not predict response to steroids or the need for secondary immunosuppression. In this retrospective study, the pattern of inflammation did not predict the need for steroids, the length of time that steroids is required, or the need for secondary immunosuppression. A cholangitic pattern was seen when the pattern of LFTs was cholestatic, and was associated with imaging abnormalities of the bile duct, but a similar pattern was seen in bile duct obstruction and other drug reactions.
Similar content being viewed by others
Background
Immune checkpoint inhibitors (ICIs) targeting cytotoxic T-lymphocyte associated antigen 4 (CTLA4), the programmed death receptor 1 (PD-1), and its ligand (PD-L1) can induce durable responses against a wide range of advanced malignancies, and have changed the therapeutic landscape in oncology [1]. Immune dysregulation resulting in immune related adverse effects, however, remains a serious limitation with these therapies. Inflammation of the liver or immune related hepatitis occurs in 1–17% of patients on single agent immune checkpoint blockade, and in as many as a quarter of patients receiving combination therapy [1,2,3,4] Some cases resolve without corticosteroid therapy, but the vast majority requires it [5, 6]. However, a subset of patients with immune related hepatitis are refractory to corticosteroids and require secondary immunosuppression. Although the impact of corticosteroids on antitumor responses has not been rigorously examined, it is likely that both corticosteroids and T-cell targeted immunosuppressive drugs have deleterious effects on antitumor responses, based on results from retrospective analyses and animal models [7,8,9]. Therefore, the ability to guide treatment of checkpoint inhibitor hepatitis by characterizing histological patterns of liver injury and identifying patients who are most likely to respond to corticosteroids could have substantial clinical impact.
Most descriptions of ICI related liver injury have focused on ICI related hepatitis. Reports of the histopathologic features in these cases describe varying degrees of lobular hepatitis with numerous histiocytes, endothelialitis, loose or well-formed granulomas, fibrin ring granulomas, and varying degrees of portal inflammation[10,11,12,13]. Rare cases present a pattern of fatty liver disease. Cases of ICI related cholangitis have also been described [12,13,14,15,16,17,18,19]. These cases may show features that overlap with sclerosing cholangitis or bile duct obstruction clinically, but the pathologic features are less well described, other than portal-based mononuclear inflammation with duct injury and perhaps cholangiolitis. Patients with ICI related cholangitis seem to respond more poorly to corticosteroid therapy.
In this study, we sought to address whether histologic pattern on liver biopsy plays a useful role in determining whether a patient’s LFTs are likely to be related to ICIs, and whether the pattern of liver injury predicts corticosteroid responsiveness. We hypothesized that liver biopsies showing more hepatitic patterns of injury were more specific for ICI related injury whereas portal-based inflammation with duct injury was less specific for ICI-related liver injury. We also hypothesized that cholangitic patterns of injury would prove steroid resistant compared to cases of inflammatory lobular hepatitis.
Methods
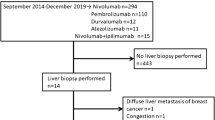
We conducted a retrospective review of 60 cancer patients treated with immune checkpoint inhibitors who underwent liver biopsy due to elevated liver function tests thought to be secondary to immune related hepatic toxicity from 2014 to 2018 at the Massachusetts General Hospital in Boston, MA. The cohort was identified from a registry of patients treated with immune checkpoint inhibitors at the Massachusetts General Hospital and by a search of the surgical pathology files for cases of immune checkpoint inhibitor induced liver injury, with the goal of procuring a consecutive series of patients who underwent liver biopsy for a suspicion of ICI related liver injury.
Histologic slides were reviewed by a single pathologist (JM) blinded to the clinical circumstances. H&E and trichrome stains were reviewed in all cases. The dimensions of the biopsy were recorded (length, number of portal tracts). The biopsies were classified as being predominantly hepatitic, cholangitic, mixed hepatitic and cholangitic, steatotic, or as nonspecific mild changes. To qualify as mixed, the biopsy had to show both significant lobular injury as well as portal inflammation with more than minimal bile duct injury and/or ductular reaction. In addition, specific histologic parameters were assessed including portal inflammation density and cell type; duct changes; lobular injury degree and zone; presence, type, and location of granulomas; presence and location of endothelialitis; the grade of steatosis, its zone, and association with steatohepatitis according to the Clinical Research Network grading system for steatohepatitis; [20] and the stage of fibrosis according to Brunt et al. [21]. Lobular injury was assessed as mild if it consisted of occasional or few foci of spotty lobular injury or singe cell necrosis, as moderate if there were either increased number or size of necroinflammatory foci, and as marked if there was confluent necrosis or zonal necrosis. We collected data from the electronic health records including age, gender, malignancy, pattern of liver enzyme abnormality, concurrent immune related adverse events, administration and type of immunosuppression, and response to immunosuppression. Risk factors for fatty liver disease were recorded, including hyperlipidemia, hypercholesterolemia, diabetes, or obesity. Because many of these patients had numerous comorbidities and their LFTs often stayed mildly elevated, we defined resolution of the acute event as a sustained reduction to grade 1 or better of the Common Terminology Criteria for Adverse Events (CTCAE) for both transaminases and alkaline phosphate (<3x ULN). If a patient did not achieve this endpoint before death, then the days to death was recorded for the purposes of calculating the median number of days to resolution. Steroid responsiveness was assessed by considering the need for steroids, the length of time of steroid use, and the need for secondary immunosuppression. Steroid use was categorized as no steroids, less than 1 month of steroids, 1–3 month of steroids, or greater than 3 months of steroids. If a patient was on steroids for a previously diagnosed condition, then whether steroid dose was increased was considered. We also collected date of death or date of last visit with a healthcare provider within the system. Follow up was censored at September 2019. In this retrospective study, we also reviewed the medical record to determine whether the final clinical impression was that the LFT abnormalities were likely to have been related to ICIs, whether there were competing explanations for the LFT abnormalities, or whether comorbidities were felt to be more likely to have been the cause for the LFT abnormalities than ICIs. However, patients were not excluded based on this final clinical impression since all of the patients underwent biopsy because ICI injury was in the differential diagnosis at the time of biopsy, the final assessment was not apparent at the time the biopsy was interpreted, and because, in some cases, the pathologic diagnosis, steroid requirements, and steroid responsiveness informed the clinical impression that ICIs were less likely to contribute to the pathologic picture.
The study was approved by the Partners Healthcare Institutional Review Board.
Results
Clinical features
Baseline characteristics of the 60 patients are shown in Table 1. Mean age at the time of liver biopsy was 61 years with a range of 28–85 years. There was a nearly even sex distribution, with 48% males, and 52% females. In total, 37 patients had at least one risk factor for fatty liver disease. The most common malignancy was melanoma (68%). Other malignancies included small numbers of nonsmall cell lung cancer, gastrointestinal adenocarcinoma, pancreaticobiliary adenocarcinoma, gynecological malignancy, acute myeloid leukemia, and cutaneous squamous cell carcinoma. One-third of patients had received combination anti-CTLA4 and anti-PD1, and another 8 patients received them sequentially. Fifteen (25%) received only anti-PD1 and only 4 received monotherapy anti-CTLA4. Thirteen patients received immune checkpoint inhibitors in combination with a non-checkpoint inhibitor agent. The median days on immune checkpoint inhibitor therapy prior to developing elevated LFTs was 78. The grade of LFT abnormality, according to the CTCAE, was most often grade 3 (73% of cases). The pattern of liver enzyme abnormality was hepatitic (50%), cholestatic (40%), or mixed (10%).
Radiologic imaging was performed for 59 of the 60 patients within the 3 months prior or one month after liver biopsy. Most patients had a CT scan only (n = 37) and another 5 had PET CT. Nine patients had a CT scan and an ultrasound of the liver whereas 3 had only an ultrasound. Five patients had either an ERCP or an MRCP, of which one had both, one had an ultrasound, one had a CT scan, and one had all 4 modalities (ERCP, MRCP, CT scan, and ultrasound). Half of the patients had no relevant liver findings. Fourteen patients had hepatic metastases, ranging from 1 to numerous nodules. Gallbladder thickening was described in 5 patients (one with metastases). Nonspecific portal or gallbladder edema was reported in 2 patients. The radiologist commented on steatosis in 5 cases and cirrhosis in 1. Dilatation of the bile duct was described in 4 patients. One of these 4 patients had imaging features suggesting cholangitis. Two of the 4 patients with dilatation had liver metastases; one had irregularity of the duct lumen centrally with presumed obstruction due to portal nodes, whereas the other had mild narrowing at the bile duct confluence. One had mildly dilated bile duct without obstructive features. Finally, one patient with liver metastases showed severe short segment narrowing of the mid common bile duct.
Thirty-two patients were already on steroids when the biopsy was procured, either due to concurrent immune mediated adverse events or concern for ICI-related liver injury; of these, 9 were on steroids 5 days or less, 6 of whom were on steroids 1 or 2 days. Twenty-eight (47%) patients required either no steroids or less than 1 month of steroids; one of these patients was on chronic replacement steroids for adrenalectomy, but the dose was not increased as a result of the new diagnosis of ICI related hepatitis. In contrast, 18 patients required more than 3 months of immunosuppression and 12 (20%) patients required a second form of immunosuppression (although 2 had immune related colitis that also required these agents). The median days to resolution of the acute event with either resolution of LFT abnormalities or returning to grade 1 according to CTCAE was 52, with a range of 2 to 302 days.
Ultimately, 13 patients were felt to have competing explanation for the LFT abnormalities. In 5 patients, disease progression in the liver was suspected of causing obstruction of the duct system, and in 2 of them, sepsis or biliary sepsis was considered a likely culprit. In 6 patients, other drugs were possible culprits, including Bactrim, a statin, carboplatin, irinotecan, temozolomide, and emactuzumab; two of these patients also had disease progression in the liver that may have contributed to the LFT abnormalities. In 1 patient, GVHD was in the clinical differential diagnosis and in the final patient, lack of compliance with lactulose was considered the cause of decompensation in a known cirrhotic.
At the time of censorship, 40% of patients were deceased from cancer. Of the remainder, half were alive without evidence of tumor on imaging and the other half were still undergoing treatment for their cancer.
Histologic features
The 60 liver biopsies ranged from 1.1 to 3.3 cm in length (mean 2.2 cm) and contained a median of 10.5 portal tracts (range 2–27). The histologic features of the biopsies are summarized in Table 2.
Twenty-eight (47%) of the 60 biopsies showed a predominantly hepatitic pattern of injury. These biopsies showed lobular injury that was mild to moderate in most cases but marked in 6 cases. The zone of injury was typically centrilobular or centrilobular predominant (17 cases; 36%) (Fig. 1) and less often azonal (8 cases) or panlobular with or without zone 3 accentuation (2 cases) (Fig. 2). Only 1 case showed a predominantly zone 1 pattern of lobular injury. In all cases, the lobular inflammation comprised mostly histiocytes with admixed lymphocytes. Five cases had scattered plasma cells and 3 cases had scattered eosinophils in the lobular inflammatory infiltrate. Mild to moderate steatosis was present in 12 cases, but in 7 cases it was confined to areas of lobular inflammation and injury (Fig. 3).
Among the 28 biopsies, 11 (39%) had granulomas, typically located in the lobules or specifically in zone 3, with only 1 case also having focal portal granulomas. The granulomas were generally loose aggregates of histiocytes, although 1 case had somewhat more well-formed granulomas and 4 cases had fibrin ring type granulomas (Fig. 4). Ten cases had endothelialitis (Fig. 5), always involving the central vein, and in 2 cases, also involving portal veins.
In the 28 hepatitic biopsies, some degree of portal inflammation was generally present as well, and usually it was mild (19 cases) but in 6 cases, the portal inflammation was at least focally moderate (Fig. 2). The portal inflammatory cells mirrored the lobular inflammation, with a mixture of mononuclear cells with or without eosinophils being the most common inflammatory milieu (21 cases; 75%). Scattered neutrophils were noted in another 6 cases. However, bile duct injury in these cases was either absent or at most minimal and did not suggest a concurrent cholangitic pattern of injury.
In 8 of the 60 cases (13%), the biopsy showed a mixed hepatocellular and cholangitic pattern of injury. In 6 of these cases, the lobular injury was moderate in severity, whereas in 2 cases, it was mild. Three cases showed a centrilobular pattern of injury, 3 were azonal, 1 was panlobular, and 1 was periportal. In these three cases, the portal inflammation was moderate, and in 2 cases it was marked. Most portal areas showed a mixed infiltrate, with mononuclear cells, scattered eosinophils, and, in 7 cases, neutrophils. In these cases, the bile ducts showed signs of injury, including intraepithelial inflammatory cells, reactive nuclear enlargement, nuclear disarray, and rarely apoptotic biliary epithelial cells. In this group, 6 biopsies had granulomas, including 1 with well-formed granulomas and 2 with fibrin ring type granulomas. However, the granulomas were limited to portal areas in 3 cases. In 2 cases, the granulomas were in both the portal areas and lobules, and in one case, only adjacent to the central veins. Endothelialitis was present in 4 cases, involving the central veins in all cases, and both central veins and portal veins in 1 case.
A predominantly cholangitic pattern of injury was found in 16 cases (26%). These cases were characterized by absent or only focal lobular injury, and instead mild to marked duct injury often accompanied by mild portal edema. Portal inflammation was graded as mild to moderate, and usually (11 cases; 69%) contained conspicuous neutrophils around injured ducts (pericholangitis) (Figs. 6 and 7). Only 2 cases showed portal inflammation that was composed of mononuclear cells with or without eosinophils. Three cases showed a mixed portal infiltrate that included mononuclear cells together with neutrophilic pericholangitis. Only one case in this group showed a granuloma adjacent to a portal tract and none showed endothelialitis. Mild to moderate steatosis was seen in 3 cases.
a Medium power view shows a portal tract that is expanded with mild edema and a mild mixed inflammatory infiltrate. b High power view of the ducts shows epithelial injury with cytoplasmic vacuolization, inflammatory cells around the duct, and, at the bottom of the field, neutrophils within the duct epithelium.
a A portal tract in a liver biopsy with a cholangitic pattern of injury, showing portal edema, bile duct injury, and scattered neutrophils around the injured ducts. Note the absence of a significant lobular inflammatory component. b High power view of a portal tract in a liver biopsy with a cholangitic pattern of injury, showing portal edema, sparse inflammation with a predominantly neutrophilic infiltrate associated with injured bile ducts demonstrating nuclear disarray and focal nuclear dropout.
Three cases (5%) had a pattern that was indistinguishable from non-alcoholic fatty liver disease, having only steatosis or a pattern of non-alcoholic steatohepatitis. One of these cases had a pattern of steatohepatitis with stage 2 of 4 fibrosis (Brunt stage) on Trichrome stain (Fig. 8). One biopsy was cirrhotic with grade 2 of 3 steatosis, without steatohepatitis. The third biopsy had grade 2 of 3 steatosis without steatohepatitis or steatofibrosis. None of them had conspicuous zone 3 necrosis, granulomas, or endothelialitis. Portal inflammation was generally absent or mild and nonspecific.
a Hematoxylin and eosin stained section. There is moderate macrovesicular steatosis, hepatocyte ballooning with Mallory-Denk bodies, and focal lobular mononuclear cell infiltrate. b Trichrome stain of the same area of the biopsy shows sinusoidal fibrosis in zone 3, as well as delicate septae extending from the portal tract, consistent with stage 2 of 4 fibrosis (Brunt stage).
Five cases (8%) had mild and nonspecific changes. These cases had absent or only focal lobular injury and absent or only focal mild portal inflammation. Three cases had focal steatosis. None of these cases had granulomas or endothelialitis.
Clinicopathologic correlation
The clinicopathologic correlation among these cases is summarized in Table 3. There was no difference between the groups in terms of the number of patients on steroids at the time of biopsy. Among the 3 patients with steatotic pattern, only 1 (the patient with grade 2 steatosis without steatohepatitis or fibrosis) was on steroids at the time of biopsy, but the steroids had been started only one day prior to biopsy. The pattern of liver enzyme abnormality correlated with the pathological pattern of injury. In patients with a hepatitic pattern of liver injury, 79% had a hepatocellular pattern of liver enzyme abnormality with elevated AST and ALT, whereas 75% of patients with a cholangitic or mixed pattern of injury on biopsy had a cholestatic pattern of liver function test abnormality with elevated alkaline phosphatase disproportionate to AST and ALT. The other 2 histological patterns did not have predictive liver enzyme abnormalities. There was no correlation between the pathologic pattern and the presence of clinical risk factors for fatty liver disease. Among 16 patients with no steatosis, 9 had at least one risk factor for fatty liver disease, and of 10 patients with grade 1 steatosis, 7 had at least one risk factor for fatty liver.
Among the 28 patients with a hepatitic pattern of liver inflammation, only one had an MRCP and none had ERCP. Nineteen (68%) of the 28 had no radiologic hepatic findings including the patient who underwent MRCP, and another 4 had only steatosis on imaging. Four patients had hepatic metastases. Only one patient had bile duct dilatation which was reportedly stable. One patient in this group had gallbladder thickening reported on ultrasound only. In this group, 17 patients had at least one risk factor for fatty liver disease, but there was no association between the presence or absence of steatosis, or the grade of steatosis, with the presence or absence of risk factors for fatty liver disease.
All 8 patients with a mixed pattern of injury had a CT scan and 2 also had ultrasound. Three patients had no radiologic abnormality related to the liver. Three patients had hepatic metastases. One patient had a thickened gallbladder on CT, and one had portal edema. Five patients had at least one risk factor for fatty liver disease, 4 of whom had no steatosis on liver biopsy. One patient had a competing clinical differential diagnosis for the LFT elevations. This was a 55 year old man with relapsed acute myelogenous leukemia, status post bone marrow transplant, on ICI therapy to boost graft versus leukemia response; therefore, GVHD was also in the differential diagnosis. The biopsy in this case showed duct injury with cytoplasmic vacuoles, but also marked portal expansion with a mixed infiltrate, numerous histiocytes, a few acidophil bodies at the portal tract interfaces, and prominent portal vein endothelialitis, more compatible with ICI related liver injury than GVHD. Steroids and immunosuppression were stared, but the patient expired 3 months later without achieving a response.
Among the 16 patients with a cholangitic pattern of inflammation on liver biopsy, ten had competing explanations for the LFT abnormalities, including disease progression in the liver (6 patients), concomitant chemotherapy (3 patients), other drugs including antibiotics (2 patients), and sepsis (2 patients). Of the 15 patients who had radiologic studies performed, only 5 patients had normal imaging findings in the liver. Seven patients had hepatic metastases (p = 0.03 when compared to the predominantly hepatitic group). Four patients had dilatation or narrowing of the bile duct (p = 0.03 compared to the predominantly hepatitic group only; p = 0.012 if the hepatitic and mixed pattern groups are combined), 3 of whom also had metastases to the liver. Two patients had gallbladder wall thickening and 1 had portal edema. Twelve of the 16 patients had at least one risk factor for fatty liver disease, 10 of whom had no steatosis on biopsy.
Among the 5 patients with mild nonspecific inflammatory changes in the liver, one had gallbladder wall thickening and the others had normal hepatic imaging. One patient had risk factors for fatty liver disease. One patient had a competing explanation for the LFTs. This patient had grade 3 elevations in the context of ICI inhibitors combined with temozolomide. The LFTs resolved to grade 1 elevations with discontinuation of medications, after which a biopsy showed minimal lobular inflammation and minimal duct injury with mild rarefaction of hepatocyte cytoplasm, consistent with a resolved process.
Among the 3 patients with a steatotic pattern on liver biopsy, steatosis was mentioned in the CT scan report in only 1 case, which resembled steatohepatitis on biopsy. One patient in the steatosis group had hepatic metastases on CT scan and 2 had risk factors for fatty liver. One patient with known cirrhosis developed large volume ascites and a CT scan reported cirrhosis and gallstones. A biopsy was performed to exclude an inflammatory cause; the biopsy showed cirrhosis with mild steatosis only.
There was no association between pattern of liver injury and requirement for long-term immunosuppression, time to resolution, or need for secondary immunosuppression. This lack of association was true even when the mixed group was combined with either the hepatitic group or the cholangitic group. The pattern of LFT elevations, whether hepatocellular, cholestatic, or mixed, did not correlate with >3 months of steroid use, or the need to add secondary immunosuppression. In the hepatitic group, there was no statistically significant association between the degree of lobular damage and the CTCAE grade of LFT elevation, the need for or length of time on steroids, or the need to add secondary immunosuppression (data now shown). This lack of a significant association between lobular damage and other parameters remained true when only patients who were not on steroids at the time of biopsy were evaluated, although the small number of patients in some groups may limit our ability to detect weak associations. Also, the presence or absence of granulomas or of endothelialitis did not predict longer time requirements for steroids or the need for secondary immunosuppression. Patients with a mild or nonspecific pattern of injury had the shortest median time to resolution, despite one patient failing to achieve this endpoint before demise; however, even after excluding this one patient, the mean days to resolution between this group and the combined hepatitis, mixed, and cholangitis groups was not statistically significantly different.
Discussion
In this study, we found that patients on immune checkpoint inhibitors have a range of pathologies on liver biopsy, including hepatitic, cholangitic, and mixed patterns of injury. The most common pattern in our series is a hepatitic pattern of injury, which was most likely related to immune checkpoint injury. A subset has a mixed hepatitic and cholangitic pattern of injury. Some patients have a purely portal-based cholangitic pattern on liver biopsy, but these patients often had alternative explanations for their elevated liver tests, including disease progression in the liver or other drugs. The pattern of liver function test abnormality correlates with the findings on liver biopsy, with a predominantly hepatocellular or cholestatic pattern of LFT abnormalities corresponding to a hepatitic or cholangitic pattern of inflammation, respectively. We found that the pattern of liver injury (whether hepatitic or cholangitic) did not predict response to steroid therapy or the need for secondary immunosuppression. Among cases with a hepatitic pattern on liver biopsy, the degree of lobular injury did not correlate with the degree of steroid responsiveness. Neither endothelialitis nor granulomas predicted the length of time needed on steroids or the need for secondary immunosuppression.
The histologic features of ICI-induced hepatitis have been described as a lobular, panlobular, or zone 3 hepatitis with numerous histiocytes, sometimes forming loose, well-formed or fibrin ring granulomas, endothelialitis, and varying portal inflammation [10,11,12,13]. In our series, we found 60% of biopsies had either predominantly hepatitic injury or mixed hepatitic and cholangitic patterns of injury. Twenty-eight of these patients had a treatment regimen that included a PD-1 inhibitor. Slightly over half of these biopsies (56%) showed a centrilobular pattern of injury and nearly a third (28%) had an azonal pattern of lobular injury. Only 4 of 36 (11%) had a panlobular pattern of injury, and 2 of 36 (6%) had a largely periportal pattern of lobular injury.
In biopsies with a significant hepatitic component, portal inflammation was also usually present, and while it was mild in most cases, it was moderate in 39%. Most cases showed portal mononuclear inflammation with scattered eosinophils but 13 (36%) of the 36 biopsies showed neutrophils together with mononuclear cells and eosinophils. In 8 of these cases, there was sufficient portal inflammation, duct injury, and ductular reaction to classify the pattern as mixed hepatocytic and cholangitic. These cases were more likely to have a cholestatic pattern of LFT abnormality.
In total, 16 cases had granulomas, including fibrin ring granulomas in 6 and well-formed granulomas in 2 cases. However, the location of the granulomas differed between the hepatitic group and the mixed hepatitic and cholangitic group, with almost all granulomas in the hepatitic group located in the lobules, but more portal-based granulomas in the mixed pattern group. Endothelialitis was present in 14 cases, with 4 showing both central vein and portal vein involvement. Steatosis was a common finding in biopsies with lobular injury, present in 44% of cases with either hepatitic or mixed patterns of injury. In 7 cases, steatosis was localized to the areas of lobular injury, a finding that was noted in prior cases in the literature [11], and suggesting that in some cases, steatosis is a component of the lobular damage rather than background steatosis. In our practice, we find that biopsies that have a hepatitic or mixed pattern of injury are the most specific appearing, and many are specifically diagnosed as ICI-related hepatitis. Only 2 of these patients, both with a mixed pattern of injury on biopsy, had a competing explanation for their elevated LFTs (cytotoxic chemotherapy administration).
The second most common pattern of inflammation in patients on ICI with elevated LFTs was portal-based inflammation without a lobular component (cholangitic pattern). Ten of these patients were given nivolumab alone or in combination with ipilimumab, and the others were given either pembrolizumab (5 cases) or atezolizumab (1 case). In our cases, this group was more likely to have imaging findings including bile duct dilatation or narrowing, although it should be noted that 3 of the 4 patients with bile duct changes also had hepatic metastases, and hepatic metastases were more common in this group relative to the hepatitis group; therefore, compression of the duct by metastatic tumor may be the cause of the liver test abnormalities and the pathologic findings. In fact, in most of these patients, bile duct obstruction from tumor or other drugs were felt to be the cause of the LFT abnormalities, often bolstered by the pathologic diagnosis rendered at the time of biopsy interpretation, which usually favored processes other than ICIs.
In the cholangitic biopsies, bile duct injury and neutrophilic pericholangitis were the dominant findings. In fact, 11 (69%) out of 16 biopsies in this group showed portal inflammation that was dominated by neutrophils around ducts or ductules. Granulomas were rare in this group and endothelialitis was not seen. Review of the patients’ biopsies showed no consistent differences between patients for whom competing causes were ultimately suspected of contributing to or causing the LFT abnormalities, and those for whom ICI were suspected of causing the abnormalities. Other reports describe cholangitis in ICI related liver injury, particularly with anti-PD-1 therapy. The cholangitis in ICI related liver injury may affect the large ducts or the small ducts. When large ducts are affected, there may be imaging findings, including sclerosing cholangitis-like changes (irregular caliber, stenoses, or thickening of the intrahepatic or proximal bile ducts) [13,14,15,16], dilatation or prominence of the extrahepatic bile ducts without a clear obstruction [17, 18], obstruction of the distal common bile duct [22], and thickening of the gallbladder [15, 17, 22]. Cases of large duct cholangitis secondary to nivolumab in which the bile duct was biopsied showed infiltration of the duct epithelium by either mixed inflammation or by neutrophils [13, 18, 22]. Histologic findings in liver biopsies in ICI related cholangitis have included a predominantly portal-based mononuclear or mixed infiltrate with duct injury, intraepithelial lymphocytes in duct epithelium, mild periportal necrosis, ductular reaction, biliary type interface activity, and cholangiolitis [12,13,14, 19]. In addition, there have been cases of vanishing bile duct syndrome in ICI related duct injury [23].
In the cases reported in the literature, ICI related cholangitis has been more resistant to steroid therapy. We did not find this in our series; in our set of patients, this pattern on liver biopsy did not predict a longer time requirement for steroids or need for secondary immunosuppression. In fact, most of our patients that did not respond well had disease progression to explain their lack of response to steroids, and for the 6 cases without competing causes for abnormal LFTs, the median days to resolution was 8 days (range 6–22 days). This discrepancy may be due to the fact that our cases might represent small duct disease. Perhaps large duct disease, with radiographic evidence of sclerosing cholangitis or duct obstruction, would portend less response to steroids. However, whenever this pattern is encountered, competing explanations for the LFT abnormalities should be sought.
In our practice, a cholangitic portal-based pattern of liver injury usually generates a differential diagnosis rather than a diagnosis of ICI related cholangitis; in many of our cases, other drug reactions, bile duct obstruction, and, depending on clinical circumstances, other causes of portal neutrophilic inflammation associated with ducts such as sepsis were favored over ICI-related cholangitis. Ultimately, in many of these cases, the LFTs were attributed to duct obstruction from tumor or other drug reactions, but the histologic features in those cases were similar to the cases of presumed ICI related cholangitis, emphasizing that portal-based inflammation with a predominance of neutrophils and injured bile ducts is not specific for ICI related liver injury.
Mild to moderate steatosis is common in biopsies of patients that are presumed to have checkpoint induced liver injury. In patients with a hepatitic pattern of injury, the fat is often localized to areas of lobular injury, but the overall appearance suggests an inflammatory lobular injury rather than nonalcoholic fatty liver disease. Most, but not all, of these patients have risk factors for fatty liver disease, even among patients with fat localized to areas of lobular injury, and it is unclear if the fat is secondary to lobular injury or bystander. In 3 of the patients in our series, the pattern of injury in the liver was indistinguishable from nonalcoholic fatty liver disease, which was described in a prior series [10]. In 1 patients, the biopsy was cirrhosis with steatosis, but this patient underwent biopsy to exclude ICI related injury due to decompensation rather than for elevated LFTs. In another patient, there was fibrosis, indicating that the fatty liver was a chronic underlying issue in this patient. In the 2 noncirrhotic cases, it is difficult to explain the rise in LFTs based on the histology. Steroid use cannot explain this pattern, as 1 of these patients was not on steroids, and the other had only been put on steroids the day before the biopsy. Five patients had very mild and nonspecific changes in their liver biopsies; although this group had the shortest median time to resolution of LFTs, there was no significant difference when compared to other groups.
A limitation of this study is its retrospective nature. In our practice, we advocate for patients to ideally undergo biopsy before treatment with steroids is implemented, but this is not always done due to concerns for severe liver injury, difficulty in arranging a liver biopsy in a timely manner, or concomitant immune mediate adverse events. Obviously, steroid use may affect the inflammatory pattern in a liver biopsy. In our cases, we were unable to detect significant differences between the patients who were and were not on steroids at the time of their biopsy. However, small numbers of patients in some groups create limitations for detecting statistical differences, and prospective analyses with early biopsy prior to initiation of any immune suppression will likely be necessary before we can make definitive judgements. Another limitation of our study is the difficulty of determining whether patients truly have ICI-related liver injury or another cause for the liver findings, particularly with the cholangitic pattern. However, this reflects the real world challenge of assigning the cause of elevated LFTs to one cause over another, in patients with numerous comorbidities and complicated oncologic courses.
In conclusion, in this retrospective study, we found that a liver biopsy in patients on immune checkpoint inhibitors can provide information on the pattern of injury, but that does not predict the need for steroids, the length of time that steroids is required, or the need for secondary immunosuppression. The degree of lobular injury did not correlate with the level of LFT abnormality or the need for steroids or secondary immunosuppression among patients with a hepatitic pattern of injury. Specific features such as granulomas and endothelialitis did not predict steroid responsiveness. A portal-based inflammatory process with duct injury and neutrophilic pericholangitis may be seen, with or without a concomitant hepatitic pattern, particularly when a cholestatic pattern of LFT elevation is present. When this pattern is the dominant one, pathologists should offer a broader differential, including ICI-related cholangitis, bile duct obstruction from tumor (especially in patients with known hepatic metastases on imaging), other drug reactions, and sepsis.
References
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Kurashima Y, Kiyono H. Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu Rev Immunol. 2017;35:119–47.
Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged survival in stage iii melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–55.
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26.
Reddy HG, Schneider BJ, Tai AW. Immune checkpoint inhibitor-associated colitis and hepatitis. Clin Transl Gastroenterol. 2018;9:180.
Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv264–iv266.
Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124:3706–14.
Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol. 2019;40:511–23.
Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–8.
Johncilla M, Misdraji J, Pratt DS, Agoston AT, Lauwers GY, Srivastava A, et al. Ipilimumab-associated hepatitis: Clinicopathologic characterization in a series of 11 cases. Am J Surgical Pathol. 2015;39:1075–84.
Everett J, Srivastava A, Misdraji J. Fibrin ring granulomas in checkpoint inhibitor-induced hepatitis. Am J surgical Pathol. 2017;41:134–7.
Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31:965–73.
Zen Y, Chen YY, Jeng YM, Tsai HW, Yeh MM. Immune-related adverse reactions in the hepatobiliary system: second-generation check-point inhibitors highlight diverse histological changes. Histopathology. 2020;76:470–80.
Hamoir C, de Vos M, Clinckart F, Nicaise G, Komuta M, Lanthier N. Hepatobiliary and Pancreatic: Nivolumab-related cholangiopathy. J Gastroenterol Hepatol. 2018;33:1695.
Kono M, Sakurai T, Okamoto K, Masaki S, Nagai T, Komeda Y, et al. Efficacy and safety of chemotherapy following Anti-PD-1 antibody therapy for gastric cancer: a case of sclerosing cholangitis. Intern Med. 2019;58:1263–6.
Gelsomino F, Vitale G, Ardizzoni A. A case of nivolumab-related cholangitis and literature review: how to look for the right tools for a correct diagnosis of this rare immune-related adverse event. Invest New Drugs. 2018;36:144–6.
Kawakami H, Tanizaki J, Tanaka K, Haratani K, Hayashi H, Takeda M, et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest N. Drugs. 2017;35:529–36.
Kuraoka N, Hara K, Terai S, Yatabe Y, Horio Y. Peroral cholangioscopy of nivolumab-related (induced) ulcerative cholangitis in a patient with non-small cell lung cancer. Endoscopy. 2018;50:E259–E261.
Gelsomino F, Vitale G, D’Errico A, Bertuzzi C, Andreone P, Ardizzoni A. Nivolumab-induced cholangitic liver disease: a novel form of serious liver injury. Ann Oncol. 2017;28:671–2.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74.
Kashima J, Okuma Y, Shimizuguchi R, Chiba K. Bile duct obstruction in a patient treated with nivolumab as second-line chemotherapy for advanced non-small-cell lung cancer: a case report. Cancer Immunol Immunother. 2018;67:61–65.
Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV, et al. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open. 2017;2:e000268.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cohen, J.V., Dougan, M., Zubiri, L. et al. Liver biopsy findings in patients on immune checkpoint inhibitors. Mod Pathol 34, 426–437 (2021). https://doi.org/10.1038/s41379-020-00653-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-00653-1
This article is cited by
-
Anti-nuclear antibody and a granuloma could be biomarkers for iCIs-related hepatitis by anti-PD-1 treatment
Scientific Reports (2022)
-
Clinicopathological analysis of hepatic immune-related adverse events in comparison with autoimmune hepatitis and graft-versus host disease
Scientific Reports (2021)
-
Clinical course of liver injury induced by immune checkpoint inhibitors in patients with advanced malignancies
Hepatology International (2021)
-
Immunotherapies/temozolomide
Reactions Weekly (2021)