Abstract
Metachronous development of intraductal papillary mucinous neoplasms in the remnant pancreas following resection is a significant clinical burden. Our aim was to characterize the clinicopathological and molecular features of the patients with metachronous tumor development to identify predictive factors and the possible route(s) of dissemination. Seventy-four patients who underwent resection of intraductal papillary mucinous neoplasms with no invasive compartment or associated carcinoma were retrospectively analyzed. In patients with metachronous tumor development, targeted sequencing of 18 genes associated with pancreatic tumorigenesis and immunohistochemical detection of four proteins (p53, SMAD4, p16, and β-catenin) were performed on both primary and metachronous tumors. The distributions of microscopic neoplastic lesions were examined at surgical margins and in apparently normal tissue apart from the primary tumor. During the median follow-up period of 52 months, 9 patients (12%) developed metachronous tumors in the remnant pancreas. Primary tumors located in the body/tail of the pancreas (odds ratio, 15; 95% confidence interval, 1.6–131) and of the pancreatobiliary type (odds ratio, 6.1; 95% confidence interval, 1.1–35.7) were identified as significant risk factors for subsequent metachronous tumor development. Eight of the nine patients shared molecular aberrations between their primary and metachronous tumors, suggesting migrations from the primary tumor to the pancreatic duct as the cause of metachronous tumor development. Our data suggest that these post-resection metachronous tumors develop by skip dissemination of the primary tumor, potentially via the pancreatic duct. The development of strategies to better predict and prevent this form of tumor progression is necessary.
Similar content being viewed by others
Introduction
Intraductal papillary mucinous neoplasms of the pancreas are a spectrum of neoplasms with varying grades of dysplasia and epithelial types [1, 2]. The incidence of metachronous tumors within the remnant pancreas is 8–21% in noninvasive intraductal papillary mucinous neoplasms [3], indicating a survival benefit (even in intraductal papillary mucinous neoplasm-associated pancreatic cancer) for microinvasive disease relative to classic pancreatic ductal adenocarcinoma [4]. To obtain better prognoses, it is crucial to manage metachronous tumor development in the context of multiple clinical issues: the feasibility of total pancreatectomy [3], the significance of the surgical margin [5, 6], and the requirement for surveillance after surgical intervention [7,8,9]. Thus, it is essential to know whether metachronous tumors are recurrences of the primary tumors or independent tumors associated with polyclonal tumor initiation. Several reports have shown that intraductal papillary mucinous neoplasms are polyclonal [10, 11] and others have demonstrated monoclonal skip progression in a subset of main-duct type tumors [12,13,14].
Within intraductal papillary mucinous neoplasms, the specific tumor types giving rise to disseminated cancerous cells, the possible routes of dissemination, and the mechanisms of cell seeding have not been fully investigated [15,16,17]. Thus, the fundamental question is how a subset of neoplastic cells releases from the primary tumor and migrates to a distant area within the organ or a specific metastatic site where the selected clones are able to regrow [18,19,20,21]. The temporal and environmental factors required for such cells to reform clinically visible tumors have not been elucidated in humans [22, 23]. Detailed clinicopathological and molecular analyses are therefore crucial to adequately address these issues. Our goal was to characterize the clinicopathological features and the clonal relatedness of metachronous tumors that arise following resection of primary lesions. Moreover, we traced related tumor cells in the surgical margins and histologically normal regions of pancreas in the resected specimens using targeted resequencing of genes associated with the pancreatic cancer [24, 25].
Methods
Patients
This study was approved by the Institutional Review Board of Teine-Keijinkai Hospital (#RIN2018-030). Written informed consent was obtained from the patients prior to enrollment for genetic analysis. Data from 122 patients who underwent surgical resection for intraductal papillary mucinous neoplasms between 2004 and 2016 at Teine-Keijinkai Hospital, diagnosed with “high-risk stigmata” or “worrisome features,” were retrospectively analyzed [26]. Patients with familial pancreatic cancer were not included in the study. Patients who underwent total pancreatectomies (n = 4) and those with <12 months of follow-up after surgical resection (n = 17) were excluded. Patients with concomitant pancreatic ductal adenocarcinoma, defined as a tumor having no histological continuity with either intraductal papillary mucinous neoplasm or an adjoining low-grade component, were excluded [27, 28]. After these exclusions, 74 patients, including those diagnosed with intraductal papillary mucinous neoplasms with no invasive compartment or associated carcinoma, remained and were enrolled (Fig. 1, Supplementary Table 1).
Patient selection flow diagram. Seventy-four patients were eligible for the study after exclusion of patients who had concomitant pancreatic ductal adenocarcinoma (n = 27), who had undergone a total pancreatectomy (n = 4), or had less than 12 months of follow-up after surgery (n = 17). During the follow-up period, recurrence was found in 14 of 74 patients, with recurrent lesions emerging in the remnant pancreas in nine (eight intraductal papillary mucinous neoplasms and one pancreatic ductal adenocarcinoma); the others showed recurrence at other sites
Histological evaluation and molecular analysis
All histological sections were examined by three pathologists (YO, TS, and TF) who identified types of epithelia (gastric, pancreaticobiliary, intestinal, or oncocytic) and grade of dysplasia (low- or high-grade) of tumor samples; this scoring was performed for patients with and without metachronous pancreatic tumors as described previously [27, 28]. Primary tumors, metachronous tumors, and microscopic neoplastic lesions in apparently normal pancreas, including at surgical margins, presumably containing either pancreatic intraepithelial neoplasias or incipient intraductal papillary mucinous neoplasms, were mapped on the resected specimens. Distances between lesions and distribution patterns of the associated neoplastic foci were assessed (i.e., cancerization of the duct versus discontinuous skip dissemination).
Immunohistochemistry for p53, SMAD4, p16, and β-catenin was performed on primary and metachronous tumors using specific antibodies (details in Supplementary Methods) [24]. Tumor mutation profiles were determined via targeted amplicon sequencing of 18 pancreatic cancer-associated genes: KRAS, TP53, SMAD4, CDKN2A, GNAS, RNF43, PIK3CA, STK11, BRAF, TGFBR1, TGFBR2, MAP2K4, ARID1A, KDM6A, SF3B1, RBM10, IDH1, and CTNNB1, using the Ion PGM System (Supplementary Methods) [24]. For patients in whom the distance between primary and metachronous tumor was more than 30 mm, microscopic neoplastic lesions in the intermediate sections also were microdissected and subjected to sequencing. Pancreatic sections on the surgical margin were analyzed for mutations in KRAS (codons 12 and 13) and GNAS (codon 201) using digital PCR (Supplementary Methods).
Statistical analyses
Odds ratios and 95% confidence intervals were calculated using a logistic regression model for risk factors associated with the development of intraductal papillary mucinous neoplasms with high-grade dysplasia or invasive carcinomas in the remnant pancreas. Statistical and bioinformatics analyses were performed using R (version 3.3.2; The R Foundation). Results with a two-sided P value < 0.05 were considered to be statistically significant.
Results
Patient characteristics
Histological grading of the 74 intraductal papillary mucinous neoplasms revealed 18 with low-grade dysplasia (24%), 38 with high-grade dysplasia (51%), and 18 intraductal papillary mucinous neoplasm-associated carcinomas (24%). Epithelial type scoring was intestinal in 32 patients (43%), gastric in 27 (39%), pancreaticobiliary in 12 (17%), and oncocytic in 3 (4%).
During the median follow-up period of 52 (range 13–154) months, 14 of the 74 patients (19%) developed metachronous lesions, and 9 (12%) developed tumors within the remnant pancreas, of which 8 (11%) were resected by remnant pancreatectomies and one was treated with chemotherapy after histological diagnosis via the main pancreatic duct, due to the patient’s poor condition (Supplementary Table 1). Histological analyses of these nine lesions in the remnant pancreas confirmed the presence of eight intraductal papillary mucinous neoplasms (three high-grade dysplasia and five associated carcinomas) and one concomitant pancreatic ductal adenocarcinoma. The remaining five patients had extrapancreatic tumors: two had peritoneal dissemination, two had metastases to the superior mesenteric plexus, and one had liver metastases.
Effects of tumor location, grade, and epithelial subtype on outcome
We then analyzed risk factors associated with the development of metachronous lesions with high-grade dysplasia or invasive carcinoma (both intraductal papillary mucinous neoplasm-associated carcinoma and classic pancreatic ductal adenocarcinoma) in the remnant pancreas (Table 1). Univariate analysis revealed that the primary tumor identified in the pancreatic body or tail (P = 0.011) or of the pancreaticobiliary type (P = 0.013) were significantly associated with metachronous neoplasms in the remnant pancreas. Multivariate analysis confirmed that primary lesions located in the pancreas body or tail (Odds ratios: 14.6; 95% confidence intervals: 1.6–131; P = 0.017) or of the pancreaticobiliary type (Odds ratios: 6.1; 95% confidence intervals: 1.05–35.7; P = 0.044) were significantly associated with metachronous development.
Of the nine patients who developed metachronous tumors in the remnant pancreas, seven tumors were diagnosed within 3 years of resection. Table 2 shows histological information for primary and metachronous tumors for these nine patients. Eight patients presented with high-grade dysplasia (n = 5) or intraductal papillary mucinous neoplasm-associated invasive carcinoma (n = 3) in their primary lesions. In two of the three patients presenting with invasive compartments associated with the primary lesion, the depth of the invasion was <2 mm, and lymphovascular invasion was not evident in all three. In addition, two patients exhibited concordant grading of their tumors, whereas the remaining six were discordant, with high-grade dysplasia in the primary but invasive lesions in the secondary tumors in four patients, and vice versa in two patients.
Two (patients 2 and 8) of the three identified invasive primary lesions recurred as intraductal tumors. One patient with low-grade dysplasia in the primary lesion developed an invasive compartment in the secondary lesion. However, all resected metachronous invasive carcinomas were determined to be node-negative except for those of patient 9, in which concomitant pancreatic ductal adenocarcinoma with regional lymph node metastasis was identified. Lastly, all specimens were negative for high-grade dysplasia or invasive carcinoma at the surgical margins of their primary lesions; however, low-grade dysplasia was identified in two patients, both continuous with the primary tumor (patients 3 and 5).
Molecular analyses of primary and metachronous tumors
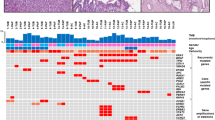
Our analysis consisted of three components: epithelial typing, immunohistochemical analyses, and targeted sequencing (Table 3, Supplementary Table 2). The types of epithelia identified within primary tumors were gastric in two patients, intestinal in three, and pancreaticobiliary in four. The epithelial types identified were concordant with their primary lesions in seven metachronous intraductal papillary mucinous neoplasm patients and discordant in one patient, in whom the primary tumor was classified as gastric and the secondary intestinal (patient 5). Immunohistochemistry revealed that aberrant expression of SMAD4 and p16 was shared between the primary and metachronous lesions of five (63%) and three (38%) of the eight metachronous patients, respectively. Nuclear accumulation of β-catenin was observed in four primary tumors. None of the lesions, neither primary nor metachronous, expressed aberrant levels of p53.
From our analysis of 18 pancreatic cancer-associated genes, all metachronous lesions had the same mutations in KRAS and GNAS present in the primary lesion (Table 3). Variants of tumor suppressor genes such as CDKN2A, SMAD4, and TP53, which are frequently mutated in classic pancreatic cancer and intraductal papillary mucinous neoplasm-associated invasive pancreatic cancers, were not detected in any tumors [24]. However, traceable mutations in STK11 (patients 2, 6, and 8), CTNNB1 (patients 4 and 7), and RNF43 (patient 1) were identified in both primary and metachronous lesions. Lastly, one metachronous pancreatic ductal adenocarcinoma in the remnant pancreas was found to harbor driver mutations completely distinct from those present in the primary lesion (patient 9; Supplementary Fig. 2).
Of the four primary intraductal papillary mucinous neoplasms with nuclear accumulation of β-catenin, three carried mutations in CTNNB1 or RNF43. However, only one recurrent tumor had the CTNNB1 S37L mutation and subsequent abnormal protein expression. CTNNB1 G34V, a pathogenic variant found in the primary lesion, was not found in the metachronous lesion, indicating that alterations in CTNNB1 do not contribute to recurrence (patient 8; Table 3, Supplementary Fig. 2). The metachronous pancreatic ductal adenocarcinoma of patient 9 stained positive for nuclear p53, although the tumor was wild type for TP53 mutations.
States of surgical margins and normal-appearing pancreases
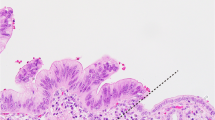
In the six patients with metachronous intraductal papillary mucinous neoplasm, no neoplastic lesions were identified at the surgical margins, in the main pancreatic duct or branch duct (Table 3). Neither KRAS nor GNAS mutations were detected by digital PCR of the margins in these patients (Supplementary Table 3). Of the two patients with a positive surgical margin at the time of resection of the primary lesion, low-grade dysplasia at the main pancreatic duct in one contained mutations identical to those of the primary and metachronous lesions (patient 5). The other patient was also characterized as having low-grade dysplasia at the main pancreatic duct, in which an identical KRAS mutation was captured by digital PCR assay in both primary and metachronous tumors, with another low-grade dysplasia in the branch duct having an additional GNAS mutation on the surgical margin (patient 3; Fig. 2).
Clinicopathological course in a patient with low-grade dysplasia in the surgical margin. Representative images and histology of primary and metachronous tumors are shown for patient 3. Yellow ovals: mural nodules in the dilated pancreatic ductal system. a A cystic mass with a solid component (arrowhead) in the pancreatic tail was resected and the main component of the intraductal papillary mucinous neoplasm was scored as high-grade dysplasia of the pancreatobiliary type (blue line). Microscopically, low-grade dysplasia was evident in the main pancreatic duct at the pancreatic cut margin (PCM; red dashed line), contiguous to the primary tumor. b Recurrent tumors emerging 2.5 years after resection histologically resembled the main component of the primary tumor (blue line). Low-grade dysplasia found at the distal end of the main pancreatic duct of the remnant pancreas (red arrow) was contiguous to the main component of the metachronous tumor (high-grade dysplasia). Identical KRAS mutations were found in the primary and recurrent lesions, including low-grade dysplasia in the PCM of the primary pancreatectomy. Scale bar: 10 mm
We further analyzed patients 6 and 7 to investigate whether the longer distance (>30 mm) between the primary and metachronous tumors in the normal-appearing pancreases was associated with the dissemination of tumor cells from the primary tumor. Microscopic neoplastic lesions were identified in the intermediate sections, and a shared KRAS variant was identified in three of four small branch-duct lesions found in patient 7, suggestive of skip dissemination (Fig. 3). In addition to the KRAS mutation, a CTNNB1 S37L mutation was identified in the microscopic lesions, further implicating distant seeding from the primary tumor via the pancreatic ductal system. On the other hand, in patient 6, identical KRAS mutations suggestive of such seeding were not observed in the microdissected samples (Supplementary Fig. 1).
Clinicopathological course before and after recurrence in a representative patient with intraductal dissemination. Multiple recurrent tumors in the pancreatic head (arrowheads) emerging 0.9 years after resection of a mixed-type intraductal papillary mucinous neoplasm with massive mural nodules in the pancreatic tail (patient 7). Both primary and recurrent lesions harbored the KRAS G12V and CTNNB1 S37L mutations. The surgical margin was negative for neoplasia; however, a series of skip lesions marked by identical mutations in KRAS and CTNNB1 with intervening normal tissue in the branch pancreatic ducts was observed. Dashed line: surgical margin. Scale bars: 1 mm
Discussion
Previous studies hypothesized that floating carcinoma cells may seed the pancreatic duct downstream of the flow of pancreatic secretions, resulting in implantation of a secondary tumor [12,13,14]. Our study supports the hypothesis that primary tumors located in the distal pancreas are more likely to develop recurrent lesions in the remnant proximal/head pancreas. Our findings indicated that even intraductal papillary mucinous neoplasms with no invasive compartment are capable of initiating implantation, resulting in metachronous tumor formation. We performed detailed pathological examinations using tissue sections prepared from entire resections, which allowed us to identify small invasive compartments (1–8 mm deep) in three patients; however, no lymphovascular or perineural infiltrations frequently associated with invasion were evident. Mutations were not observed in any of the primary intraductal papillary mucinous neoplasms in genes encoding the tumor suppressors p16, SMAD4, and p53, which are characteristic of KRAS-induced pancreatic cancer precursors and invasive properties. Thus, the tumors observed in this study may have had less-aggressive phenotypes [29, 30]. Alternatively, mutations in STK11 and CTNNB1 were enriched in a small portion of our cohort. This may be a unique genetic profile that can promote migration through the pancreatic duct system [31, 32].
We demonstrated that the most common histological type of recurrent neoplasms was associated with intraductal papillary mucinous neoplasms (high-grade dysplasia and invasive carcinoma; 8/9, 89%) rather than concomitant pancreatic ductal adenocarcinoma, which harbored driver mutations distinct from those of the corresponding primary lesions. By genotyping each tumor compartment, Pea et al. classified the development of metachronous neoplasms in patients with intraductal papillary mucinous neoplasms into three mechanisms: residual microscopic disease at the resection margin, intraparenchymal spread of neoplastic cells, and multifocal disease with genetically distinct lesions [33]. Accordingly, we demonstrated that five of the seven metachronous intraductal papillary mucinous neoplasms in our study had molecular profiles (e.g., mutation profiles of driver and traceable genes) identical to those of primary tumors with negative surgical margins, strongly suggesting spreading by seeding and skip dissemination.
Pathological examination of the resected specimens from dysplastic lesions surrounding the primary neoplasms indicated intraductal spread, with low-grade dysplasia toward the surgical margin of the main pancreatic duct observed in two patients. Genetic analysis of low-grade dysplasia using digital PCR demonstrated identical KRAS mutations to those present in the primary lesion. Skip dissemination was clearly evident in patient 7. Therefore, movement of tumor cells in the pancreatic ductal system appears to be a more frequent cause of metachronous tumor development within the remnant pancreas than intraductal continuous cancer extensions [34].
The metachronous tumor can more frequently merge following resection of intraductal papillary mucinous neoplasm primarily developed in the pancreatic body and tail. The direction of dissemination of tumor cells through the pancreatic duct is consistent with the physiological secretory flow. However, studies by our group and others have demonstrated metachronous tumor development against the direction of secretion with identical driver mutations in cases of pancreatic cancer [35, 36]. Therefore, our observations of patient 4 in this study were not surprising.
Aside from the specific location of the primary intraductal papillary mucinous neoplasm being in the pancreas body or tail, we determined that pancreaticobiliary subtype was predictive for local recurrence. Similarly, another study demonstrated that a broad spectrum of epithelial types is found in intraductal papillary mucinous neoplasm synchronous lesions and metachronous tumors following resection, with the time required for recurrence following resection longer than in this study, with a median of 5 (range 2–7) years [14]. In our study, three of the seven patients with genotype-matched tumors (43%) relapsed and were diagnosed with the pancreaticobiliary subtype. We could not, however, quantify the relative risk of recurrence within the same epithelial type due to lack of data from nonrecurrent patients.
A specific subset of tumor cells may develop the ability to grow and spread as skip lesions. Notably, the pancreaticobiliary subtype is known to be associated with the highest risk of the four epithelial types [37]; we previously demonstrated that highly dysplastic lesions are frequently located in mural nodules in this subtype [38]. Although the molecular mechanism underlying the more aggressive phenotype is still unclear, a recent study demonstrated that pancreatobiliary type IPMN is associated with PTEN inactivation, which leads to hyperactivation of the ERK signaling [39]. It is therefore important to identify biomarkers that can be exploited for use as noninvasive diagnostics. In addition to mutation profiling, gene expression, and epigenetic signatures may better describe which tumor types may disseminate aggressively among the intraductal papillary mucinous neoplasms.
A limitation of the present study is that its molecular landscape was constructed based on data from only 18 genes and a small sample size, giving our study low statistical power. Furthermore, assessment of the clonal relatedness between primary and recurrent tumors that contain shared KRAS and GNAS variants alone could not be considered to be absolute, since an incidental match can occur by chance, albeit at low probability [25]. Thus, a comprehensive mutational analysis of primary intraductal papillary mucinous neoplasms, with or without metachronous disease, may provide a more accurate genetic profile for tumors that efficiently disseminate via the pancreatic duct.
Another limitation is that patients with tumor recurrence in alternate sites following resection of the primary intraductal papillary mucinous neoplasm were not subjected to genetic analysis because of their advanced disease; therefore, our findings may only be representative of patients with less-aggressive disease. The term of post-operative follow-up was short, only including secondary tumors that recurred within 5 years. Independent metachronous disease is likely to occur more frequently in the remnant pancreas if we extend the term of post-operative follow-up or do not decide to perform a second surgery. Another concern is that our cohort comprised patients from only a single institution. Finally, we performed endoscopic retrograde pancreatography as a diagnostic procedure for pancreatic juice cytology and pancreatic duct biopsy, which itself may have affected the natural migration of disseminated and preimplanted tumor cells. A multicenter study using a larger number of patients and a full mutational profile is necessary to further clarify these issues.
To conclude, metachronous tumors following resection of intraductal papillary mucinous neoplasms may develop from skip dissemination of the primary tumor, potentially via the pancreatic duct. The development of diagnostic, therapeutic, and predictive strategies to prevent this form of tumor progression is therefore necessary.
References
Patra KC, Bardeesy N, Mizukami Y. Diversity of precursor lesions for pancreatic cancer: the genetics and biology of intraductal papillary mucinous neoplasm. Clin Transl Gastroenterol. 2017;8:e86.
Klöppel G, Basturk O, Schlitter AM, Konukiewitz B, Esposito I. Intraductal neoplasms of the pancreas. Semin Diagn Pathol. 2014;31:452–66.
Griffin JF, Poruk KE, Wolfgang CL. Is it time to expand the role of total pancreatectomy for IPMN? Dig Surg. 2016;33:335–42.
Waters JA, Schnelldorfer T, Aguilar-Saavedra JR, Chen JH, Yiannoutsos CT, Lillemoe KD, et al. Survival after resection for invasive intraductal papillary mucinous neoplasm and for pancreatic adenocarcinoma: a multi-institutional comparison according to American Joint Committee on Cancer Stage. J Am Coll Surg. 2011;213:275–83.
Dhar VK, Merchant NB, Patel SH, Edwards MJ, Wima K, Imbus J, et al. Does surgical margin impact recurrence in noninvasive intraductal papillary mucinous neoplasms? A multi-institutional study. Ann Surg. 2018;268:469–78.
Frankel TL, LaFemina J, Bamboat ZM, et al. Dysplasia at the surgical margin is associated with recurrence after resection of non-invasive intraductal papillary mucinous neoplasms. HPB (Oxf). 2013;15:814–21.
Kang MJ, Jang JY, Lee KB, D’Angelica MI, DeMatteo RP, Fong Y, et al. Long-term prospective cohort study of patients undergoing pancreatectomy for intraductal papillary mucinous neoplasm of the pancreas: implications for postoperative surveillance. Ann Surg. 2014;260:356–63.
Marchegiani G, Mino-Kenudson M, Ferrone CR, Morales-Oyarvide V, Warshaw AL, Lillemoe KD, et al. Patterns of recurrence after resection of IPMN: who, when, and how? Ann Surg. 2015;262:1108–14.
He J, Cameron JL, Ahuja N, Makary MA, Hirose K, Choti MA, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg. 2013;216:657–67.
Izawa T, Obara T, Tanno S, Mizukami Y, Yanagawa N, Kohgo Y. Clonality and field cancerization in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2001;92:1807–17.
Matthaei H, Norris AL, Tsiatis AC, Olino K, Hong SM, dal Molin M, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:326–33.
Tamura K, Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Ideno N, et al. Assessment of clonality of multisegmental main duct intraductal papillary mucinous neoplasms of the pancreas based on GNAS mutation analysis. Surgery. 2015;157:277–84.
Tamura K, Ohtsuka T, Ideno N, Aso T, Shindo K, Aishima S, et al. Treatment strategy for main duct intraductal papillary mucinous neoplasms of the pancreas based on the assessment of recurrence in the remnant pancreas after resection: a retrospective review. Ann Surg. 2014;259:360–8.
Date K, Ohtsuka T, Fujimoto T, Tamura K, Kimura H, Matsunaga T, et al. Molecular evidence for monoclonal skip progression in main duct intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2017;265:969–77.
Habuchi T, Takahashi R, Yamada H, Kakehi Y, Sugiyama T, Yoshida O. Metachronous multifocal development of urothelial cancers by intraluminal seeding. Lancet. 1993;342:1087–8.
Morales-Oyarvide V, Mino-Kenudson M. Tumor islands and spread through air spaces: distinct patterns of invasion in lung adenocarcinoma. Pathol Int. 2016;66:1–7.
Sanli O, Dobruch J, Knowles MA, Burger M, Alemozaffar M, Nielsen ME, et al. Bladder cancer. Nat Rev Dis Prim. 2017;3:17022.
Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–26.
Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61.
Pandya P, Orgaz JL, Sanz-Moreno V. Modes of invasion during tumour dissemination. Mol Oncol. 2017;11:5–27.
Makohon-Moore AP, Matsukuma K, Zhang M, Reiter JG, Gerold JM, Jiao Y, et al. Precancerous neoplastic cells can move through the pancreatic ductal system. Nature. 2018;561:201–5.
Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7.
Hosseini H, Obradovic MM, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540:552–8.
Omori Y, Ono Y, Tanino M, Karasaki H, Yamaguchi H, Furukawa T, et al. Pathways of progression from intraductal papillary mucinous neoplasm to pancreatic ductal adenocarcinoma based on molecular features. Gastroenterology. 2019;156:647–61.e2.
Felsenstein M, Noe M, Masica DL, Hosoda W, Chianchiano P, Fischer CG, et al. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut. 2018;67:1652–62.
Tanaka M, Fernandez-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–53.
Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571–80.
Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, et al. A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–41.
Qian ZR, Rubinson DA, Nowak JA, Morales-Oyarvide V, Dunne RF, Kozak MM, et al. Association of alterations in main driver genes with outcomes of patients with resected pancreatic ductal adenocarcinoma. JAMA Oncol. 2018;4:e173420.
Yachida S, White CM, Naito Y, Zhong Y, Brosnan JA, Macgregor-Das AM, et al. Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long-term survivors. Clin Cancer Res. 2012;18:6339–47.
Sato N, Rosty C, Jansen M, Fukushima N, Ueki T, Yeo CJ, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017–22.
Sethi V, Giri B, Saluja A, Dudeja V. Insights into the pathogenesis of pancreatic cystic neoplasms. Dig Dis Sci. 2017;62:1778–86.
Pea A, Yu J, Rezaee N, Luchini C, He J, Dal Molin M, et al. Targeted DNA sequencing reveals patterns of local progression in the pancreatic remnant following resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2017;266:133–41.
Eguchi H, Ishikawa O, Ohigashi H, Sasaki Y, Yamada T, Nakaizumi A, et al. Role of intraoperative cytology combined with histology in detecting continuous and skip type intraductal cancer existence for intraductal papillary mucinous carcinoma of the pancreas. Cancer. 2006;107:2567–75.
Imai K, Karasaki H, Ono Y, Sasajima J, Chiba S, Funakoshi H, et al. Metachronous pancreatic cancer originating from disseminated founder pancreatic intraductal neoplasias (PanINs). J Pathol Clin Res. 2015;1:76–82.
Luchini C, Pea A, Yu J, He J, Salvia R, Riva G, et al. Pancreatic cancer arising in the remnant pancreas is not always a relapse of the preceding primary. Mod Pathol. 2019;32:659–65.
Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509–16.
Karasaki H, Mizukami Y, Tokusashi Y, Koizumi K, Ishizaki A, Imai K, et al. Localization of the most severely dysplastic/invasive lesions and mucin phenotypes in intraductal papillary mucinous neoplasm of the pancreas. Pancreas. 2011;40:588–94.
Kopp JL, Dubois CL, Schaeffer DF, Samani A, Taghizadeh F, Cowan RW, et al. Loss of Pten and activation of Kras synergistically induce formation of intraductal papillary mucinous neoplasia from pancreatic ductal cells in mice. Gastroenterology. 2018;154:1509–23.e5.
Acknowledgements
This work was supported by JSPS KAKENHI grant number 17K09472, the Suzuken Memorial Foundation, and the Suhara Memorial Foundation, all to YM. We thank Takuya Sugimura (Teine-Keijinkai Hospital) for tissue sample preparation, and Munehiko Ogata and Atsuko Sumi (Sapporo Higashi Tokushukai Hospital) for technical support in the genetic analyses. We thank the members of the Center for Gastroenterology at Teine-Keijinkai Hospital and laboratory staff of the Institute of Biomedical Research at Sapporo Higashi Tokushukai Hospital for their helpful suggestions. We thank Helena Akiko Popiel for editorial review of the manuscript. We thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
YM and YO receive funding from Hitachi Hightechnologies Inc. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nagai, K., Mizukami, Y., Omori, Y. et al. Metachronous intraductal papillary mucinous neoplasms disseminate via the pancreatic duct following resection. Mod Pathol 33, 971–980 (2020). https://doi.org/10.1038/s41379-019-0405-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0405-7
This article is cited by
-
Case report: composite pancreatic intraductal papillary mucinous neoplasm and neuroendocrine tumor: a new mixed neuroendocrine-non-neuroendocrine neoplasm?
Diagnostic Pathology (2021)
-
The genetics of ductal adenocarcinoma of the pancreas in the year 2020: dramatic progress, but far to go
Modern Pathology (2020)
-
Time-saving method for directly amplifying and capturing a minimal amount of pancreatic tumor-derived mutations from fine-needle aspirates using digital PCR
Scientific Reports (2020)