Abstract
Renal biopsy is the gold standard for diagnosis of membranous nephropathy. Circulating PLA2R1 antibody found in 75% of patients with membranous nephropathy is very specific for the diagnosis of this disease. Therefore, the question arises whether PLA2R1-antibody-positive patients still need a diagnostic renal biopsy. In this study we investigated whether additional relevant information is obtained by performing renal biopsy in nephrotic patients, who are PLA2R1-antibody positive. A detailed analysis of renal biopsies, including immunohistochemistry and electron microscopy, was performed in 263 patients with biopsy-proven membranous nephropathy, of whom 194 patients were PLA2R1-antibody positive, to detect diagnostic features additional to membranous nephropathy. Twelve (6%) of the 194 PLA2R1-antibody-positive patients had a second relevant diagnosis in addition to membranous nephropathy: five (3%) patients had interstitial nephritis, in five (3%) other patients a diabetic nephropathy was diagnosed and two (1%) patients had IgA nephropathy. Patients with a second diagnosis in addition to membranous nephropathy had a significantly higher serum creatinine (p < 0.01) and lower eGFR (p = 0.04) compared to patients in whom only the diagnosis of membranous nephropathy was made. In 7 (10%) of 69 PLA2R1-antibody-negative patients, renal biopsies showed an additional diagnosis to membranous nephropathy: one (1%) case of IgA nephropathy, cholesterol emboli, IgG4-related disease, necrotising glomerulonephritis, thrombotic microangiopathy, interstitial nephritis and diabetic nephropathy each. The advantage of detecting an additional diagnosis to membranous nephropathy in 6% of PLA2R1-antibody-positive patients by renal biopsy has to be balanced to the potential risks and costs of the biopsy procedure. Renal biopsy is particularly relevant in patients presenting with impaired renal function and abnormalities in urinalysis going beyond proteinuria. Immunohistochemical staining for PLA2R1 was the only histomorphologic analysis allowing a reliable differentiation of PLA2R1-antibody-positive from PLA2R1-antibody-negative membranous nephropathy.
Similar content being viewed by others
Introduction
Renal biopsy is still the gold standard for the diagnosis of membranous nephropathy. A granular positivity for IgG along the glomerular basement membrane and electron-dense deposits restricted to the subepithelial space of the glomerular filtration barrier are the characteristic morphologic features [1]. By light microscopy the capillary walls are mostly thickened, however, this may be absent in early stages of disease. The identification of the phospholipase A2 receptor 1 (PLA2R1) as the major target antigen in membranous nephropathy has led to major improvements in the diagnosis and treatment of these patients [2]. The diagnosis of PLA2R1-associated membranous nephropathy can be made by measuring circulating PLA2R1-antibody levels or staining for the PLA2R1 antigen in the renal tissue [3, 4]. Staining of renal tissue is more sensitive, since PLA2R1-antibody can become negative spontaneously, or when patients are treated with immunosuppression [5,6,7]. Findings from renal biopsies are helpful not only for confirming the diagnosis membranous nephropathy but also allowing an estimation of tissue damage, i.e. tubular atrophy and interstitial fibrosis or glomerular sclerosis, as well as diagnosis of lesions not related to membranous nephropathy. Renal biopsy, however, is an invasive procedure and complications, most notably bleeding, are a matter of concern [8, 9], particularly in patients who are at high risk for bleeding. Therefore, the risks and benefits of renal biopsy have to be considered when deciding whether to perform this procedure. PLA2R1-antibody detection in serum of nephrotic patients has been shown to be extremely specific for the diagnosis of membranous nephropathy [6, 7]. Considering these points the question arises, whether nephrotic patients who are PLA2R1-antibody positive but otherwise show no symptoms or abnormalities in renal function and urinalysis need a renal biopsy to make the diagnosis of PLA2R1-associated membranous nephropathy, or whether other findings in renal biopsies of such patients might justify performing the procedure despite detection of PLA2R1 antibodies.
In order to address this question, we analysed renal biopsies, PLA2R1-antibody findings and the clinical presentation of 263 patients who were diagnosed with membranous nephropathy.
Materials and methods
Patients and study design
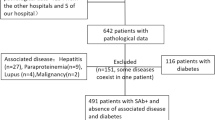
We included 263 patients with the histologic diagnosis of membranous nephropathy in this study. Patients with evidence of a secondary form of disease (i.e. lupus erythematodes) were excluded from the study. In all patients PLA2R1-antibody levels were measured within 6 months from the time of renal biopsy. PLA2R1-antibody measurement in the serum was performed by ELISA, an indirect immunofluorescence test, and western blot as described previously [3, 7, 10]. According to the manufacturer, the ELISA results were considered positive at a PLA2R1-antibody level >20 U/ml. The indirect immunofluorescence test is commercially available (EUROIMMUN, Lübeck) and makes use of transfected HEK293 cells, which express PLA2R1 on the surface. Upon incubation with a PLA2R1-antibody-positive blood sample the cells show a specific cytoplasmic fluorescence. A blood sample was considered to be PLA2R1-antibody positive if a specific cytoplasmic fluorescence of the transfected cells was detected at a serum dilution of 1:10 or higher. Some of the clinical data of this patient cohort have been published earlier [10]. Proteinuria and serum creatinine were analysed for all patients at the time of study inclusion. Membranous nephropathy was diagnosed by renal biopsy at the Nephropathology Section, Institute of Pathology, University Medical Centre Hamburg-Eppendorf. Diagnosis was made based on light microscopy, immunohistochemistry and electron microscopy findings. The study was approved by the local ethics committee of the chamber of physicians in Hamburg and conducted in accordance with the ethical principles stated by the Declaration of Helsinki. An informed consent was obtained from all participating patients.
Histological and immunohistochemical analyses
Slides with 1–2 µm thin slices from formalin-fixed, paraffin-embedded renal biopsies were stained with PAS and trichrome (Masson-Goldner-Elastica). Immunohistochemical analyses were routinely performed for IgG, IgA, IgM, C1q, C3 and fibrinogen/fibrin. After deparaffinization, slides were pre-treated in proteinase (protease P-8038, Sigma-Aldrich, St. Louis, MO, USA) at 40 °C for 15 min followed by incubation with normal serum (Vector S2000) for 10 min. Primary antibodies for IgA (1:8000), IgM (1:3000), fibrinogen/fibrin (1:12000), C3 (1:3000), C1q (1:1500) (all polycloncal antibodies from rabbit, A0262, A0425, A0080, A0062, A0136, respectively, DAKO, Eching, Germany) and IgG (1:7500) (mouse monoclonal antibody, 209-005-088, Dianova, Hamburg, Germany) were added for 30 min at 40 °C. Bound antibodies were then visualised manually using a standard APAAP protocol.
For PLA2R1 immunohistochemical analyses slides were deparaffinized, pre-treated in citrate buffer (pH 6.2) for 15 min at 120 °C and cooled down in iced water (10 min). After rinsing in 99% ethanol, slides were incubated for 10 min with normal serum (Vector S2000; Vector Laboratories, Burlingame, CA) followed by PLA2R1-antibodies (polyclonal antibody from rabbit, 1:3000, HPA 012657, Sigma-Aldrich, St. Louis, MO) overnight at 4 °C. The slides were then washed in PBS, incubated with polymer 1 (Zytomed Zytochem-Plus AP Polymer-Kit POLAP), rinsed in PBS and incubated with polymer 2 (Zytomed Zytochem-Plus AP Polymer-Kit POLAP). After washing in PBS, slides were stained in new fuchsin naphthol As-Bi phosphate substrate mixture (30 min) followed by 1 min of nuclear staining in hemalaun (Mayer).
Electron microscopy
Small parts (1 mm) of the formaldehyde fixed biopsy were put in sodium cacodylate buffer (10 min at 80 °C) and 1% osmium tetroxide and sucrose (2 h). After washing in cacodylate buffer and 1 h contrasting in uranyl acetate, the specimens were dehydrated in an ascending ethanol series and diethyl ether, embedded in araldite and polymerised 12 h up to 100 °C. Semi-thin sections were stained with toluidine blue. Ultra-thin sections were contrasted with lead citrate. Images were taken using a transmission electron microscope (Zeiss EM109) equipped with a digital camera (TRS 2K-CCD).
Statistical analyses
Descriptive analyses of continuous data are presented as median and 1st and 3rd quartile unless stated otherwise. For categorical data, absolute counts and percentages are reported. Mann–Whitney U tests were employed for group-wise comparisons of continuous and ordinal variables while Fisher’s exact tests were used for group-wise comparisons of categorical variables. Statistical significance was defined as p < 0.05.
Results
Clinical baseline characteristics
At study inclusion median serum creatinine was 1.0 mg/dl (1st to 3rd quartile 0.8–1.4 mg/dl) and proteinuria 6.3 g/day (1st to 3rd quartile 3.9–10.0 g/day) (Table 1). The median age was 56.0 years (1st to 3rd quartile 43.0–68.0 years) and 182 (69%) patients were male. The median time from renal biopsy to study inclusion and measurement of the PLA2R1-antibody levels was 0.5 months (1st to 3rd quartile 0.0–1.0 months). At study inclusion PLA2R1 antibody was detectable in 194 (74%) patients. PLA2R1-antibody-positive patients were more often males and had a higher proteinuria at baseline compared to patients who were PLA2R1-antibody negative, while age and renal function was not significantly different between the two groups (Table 1).
Renal diagnoses in biopsies from PLA2R1-antibody-positive and PLA2R1-antibody-negative patients
In 12 (6%) PLA2R1-antibody-positive patients renal biopsy revealed a second diagnosis, in addition to membranous nephropathy. Five patients had an interstitial nephritis (Fig. 1a, b), five patients had diabetic nephropathy (Fig. 1c, d) and two patients IgA nephropathy (Fig. 1e, f). Diabetes was known from clinical data in the five patients with diabetic nephropathy. Patients in whom a second renal diagnosis was made, had significantly higher serum creatinine levels (p = 0.006), lower eGFR (p = 0.04) and higher proteinuria (p = 0.001) compared to patients with the diagnosis of membranous nephropathy only (Table 2). Remarkably only 2 (17%) out of 12 patients with a second diagnosis presented with serum creatinine below 1.0 mg/dl at baseline (both patients with IgA nephropathy), while this was the case in 94 out of 182 (52%) patients with membranous nephropathy as the sole diagnosis (p = 0.03). Therefore, in our study cohort the prevalence of a second diagnosis in addition to membranous nephropathy was significantly higher in patients with serum creatinine levels above 1.0 mg/dl compared to patients with serum creatinine below 1.0 mg/dl (10% versus 2%, p = 0.03). Not surprisingly, patients with a second diagnosis in the renal biopsy also had more advanced tubular atrophy/interstitial fibrosis and glomerular sclerosis compared to patients with the diagnosis of membranous nephropathy only (Table 2). Arteriolosclerosis was also more advanced in patients with an additional diagnosis in the renal biopsy.
Biopsy findings in PLA2R1-antibody-positive patients with an additional renal diagnosis. a, b Interstitial nephritis (a PAS, b IgG immunohistochemistry); c d diabetic glomerulosclerosis (a PAS, b IgG). Concurrent IgA nephropathy with mesangial IgA deposits (e) and granular IgG deposits along the peripheral basement membranes (f, same glomerulus as e)
In PLA2R1-antibody-negative patients a second diagnosis additional to membranous nephropathy was made in 7 (10%) cases, however, the spectrum of diagnoses was markedly broader, including one case of cholesterol emboli, IgG4-related disease, necrotising glomerulonephritis, thrombotic microangiopathy, IgA nephropathy, interstitial nephritis and diabetic nephropathy each (Fig. 2). Similar to the findings in PLA2R1-antibody-positive cases, patients in whom a second renal diagnosis was made, had more advanced tubular atrophy/interstitial fibrosis and glomerular sclerosis compared to patients with the diagnosis of membranous nephropathy only (Table 3). Patients with no additional diagnosis in the renal biopsy had more often advanced stages of glomerular lesions in electron microscopy.
Biopsy findings in PLA2R1-antibody-negative patients with an additional renal diagnosis. a Cholesterol embolus in an arteriole (PAS); b interstitial nephritis (PAS); c thrombotic microangiopathy with fresh fibrin thrombi within the glomerular capillary lumen (PAS); d necrotising glomerulonephritis (PAS); e, f concurrent IgA nephropathy with mesangial IgA deposits (e) and granular IgG deposits along the peripheral basement membranes (f, same glomerulus as e)
Histomorphological findings in PLA2R1-antibody-positive patients compared to PLA2R1-antibody-negative patients
Next, we performed a detailed analysis of all renal biopsies from the patients included in the study (Table 4). In all 194 PLA2R1-antibody-positive patients a membranous nephropathy could be diagnosed in the renal biopsy. In patients with PLA2R1-antibody-positive membranous nephropathy, we found more tubular atrophy/interstitial fibrosis (p = 0.008), although the absolute difference between the two groups was small (8% versus 5% of the tubulointerstitial space) and therefore its clinical significance unclear. The extent of glomerular scarring was not different between the two groups and we found no difference in the number of biopsies showing a granular positivity for IgA, IgM or C1q along the glomerular basement membrane between PLA2R1-antibody-positive and PLA2R1-antibody-negative patients. Interestingly, patients with PLA2R1-antibody-positive membranous nephropathy were more often positive for C3 and fibrin in immunohistochemical analyses (p < 0.001 for both comparisons) and showed in electron microscopy more often advanced stages of glomerular lesions compared to PLA2R1-antibody-negative patients (p = 0.007).
Previous studies have shown that a PLA2R1 staining might have a higher sensitivity for the diagnosis of PLA2R1-associated membranous nephropathy compared to the detection of PLA2R1-antibodies in blood [3, 4]. For this reason, we performed a PLA2R1 staining in 40 out of the 69 biopsies from the patients who were negative for PLA2R1-antibodies in the serum. In 5 (13%) out of these 40 cases, we detected an enhanced staining for PLA2R1, typical for the diagnosis of PLA2R1-associated membranous nephropathy. None of these five biopsies showed a second diagnosis in addition to PLA2R1-associated membranous nephropathy.
We also performed a PLA2R1 staining in 122 out of the 194 biopsies from PLA2R1-antibody-positive patients. In 120 (98%) of these cases we were able to detect an enhanced PLA2R1 staining in the renal tissue. In the remaining two cases PLA2R1 staining was not enhanced, however, at the same time both cases showed a barely detectable positivity for IgG in the immunohistochemical staining and only very few and small electron-dense deposits in the electron microscopy. PLA2R1 antibody in both cases was only detectable by western blot, which has been shown to be more sensitive than the other serological tests (immunofluorescence test and ELISA).
Histomorphological findings in biopsies performed during the study follow-up
During the study follow-up a second renal biopsy was performed in 15 patients. The median time between the first diagnostic biopsy and the second biopsy performed during follow-up was 39 months (1st to 3rd quartile: 20–60 months). Twelve (80%) of these patients were male (median age at diagnosis 49.8 years). Ten (67%) of the 15 patients had a PLA2R1-antibody-positive membranous nephropathy while the remaining five patients were PLA2R1-antibody negative. In all 15 biopsies the diagnosis membranous nephropathy was confirmed and in none of the ten PLA2R1-antibody-positive patients an additional renal diagnosis was made at the second renal biopsy. The biopsy of one of the PLA2R1-antibody-negative patients revealed an interstitial nephritis while the remaining four biopsies did not show a second renal diagnosis.
Discussion
Renal biopsy is the cornerstone and gold standard for diagnosis of most renal diseases [11]. In adult patients with nephrotic syndrome a biopsy is needed to make the diagnosis and estimate the prognosis of the patients. In contrast, children with nephrotic syndrome are often treated with steroids without performing a renal biopsy [12]. The rationale of this approach is the fact that in almost 90% of cases renal biopsy would show minimal change disease as the cause for steroid-sensitive nephrotic syndrome. Since in these cases the risks for performing a renal biopsy are considered to outweigh the benefits of the procedure, children are treated with steroids and only if they do not respond to treatment a renal biopsy is performed. In the past few years there have been major improvements in our understanding of the pathomechanisms leading to membranous nephropathy based upon the identification of PLA2R1 as the major target antigen [2]. Detection of PLA2R1 antibody in serum has an almost 100% specificity for the diagnosis of PLA2R1-associated membranous nephropathy [3, 4], leading to the question, whether all nephrotic patients with detectable PLA2R1 antibody in serum need a renal biopsy to confirm the diagnosis of membranous nephropathy. In order to address this question, the potential risks of renal biopsy should be weighed against the potential benefits, i.e. diagnostic and prognostic information gained.
Regarding the diagnostic role of the biopsy, we found a second renal diagnosis in addition to membranous nephropathy in 12 (6%) PLA2R1-antibody-positive patients. Diabetic nephropathy was found in five cases, who were known to have diabetes mellitus. The diagnosis of additional diabetic nephropathy was therefore not surprising. However, in these cases it is likely that proteinuria is at least partially caused by the diabetic glomerulosclerosis, which could explain persistent proteinuria in patients with disappearing PLA2R1 antibody in serum in the further course of disease. Importantly, a second diagnosis was mostly made in patients who had an impaired renal function, with the exception of the two patients with IgA nephropathy. Therefore, in our study cohort the probability of a second diagnosis in addition to membranous nephropathy was significantly higher in patients with impaired renal function compared to patients with low serum creatinine levels.
During the study follow-up a second biopsy was performed in 15 patients, confirming the diagnosis membranous nephropathy in all cases. However, in none of the PLA2R1-antibody-positive patients the biopsy yielded new diagnostic information. These data suggest that patients with a PLA2R1-antibody-positive membranous nephropathy, who have a relapse of PLA2R1-antibody and relapse of proteinuria should not receive a renal biopsy to confirm the diagnosis “relapse” of disease. Such a biopsy might be warranted in cases when the pathology of disease is unclear, i.e. patients with a relapse of proteinuria but negative PLA2R1-antibody, or patients with a glomerular haematuria and acute renal failure with no obvious clinical explanation.
Renal biopsy also provides significant information in assessing the extent of chronic renal damage i.e. glomerulosclerosis, tubular atrophy/interstitial fibrosis, or arteriolosclerosis, all of which can of course be significantly present in patients with normal levels of serum creatinine. Thus, an initial biopsy can be referred to in the further course of disease and comparison to later biopsies allows estimation of the progress of chronic changes. Furthermore, electron microscopic stages of the disease can provide an indication of the time course of the immunological process. We did not perform immunohistochemical staining for kappa and lambda light chains in our cohort. Since very rare cases of membranous nephropathy can also show monoclonal appearing subepithelial deposits [13], these analyses might be diagnostically relevant in some clinical settings.
The decision to perform a renal biopsy remains to be made individually for any patient, depending on the clinical data and risk factors. In this context, the benefits of a renal biopsy should be considered against the potential risks and costs of the procedure. The risks of ultrasound-guided renal biopsy have decreased in the past few years, but they remain high in patients with a single kidney, anatomical abnormalities, or high risk of bleeding, i.e. thrombocytopenia, coagulopathy, antiplatelet or anticoagulant therapy. The data obtained from our study might be helpful for clinicians when deciding the best diagnostic approach in these patients, especially when they present with a nephrotic syndrome only, without other abnormalities in renal function and in the urine.
In PLA2R1-antibody-negative patients a renal biopsy is needed to make a diagnosis. In our cohort 13% of these patients turned out to have an enhanced immunohistochemical glomerular positivity for PLA2R1 and were therefore diagnosed as PLA2R1-associated membranous nephropathy. The higher sensitivity of the PLA2R1 staining in the biopsy compared to the PLA2R1-antibody detection in the blood for the differential diagnosis of PLA2R1-associated membranous nephropathy has been shown before [3, 4]. Our findings confirm these data and underline the diagnostic value of the kidney biopsy, which might lead to the diagnosis of PLA2R1-associated membranous nephropathy in patients who are negative for PLA2R1-antibody. In our cohort of PLA2R1-antibody-positive patients we identified two (2%) patients, in whom no enhanced PLA2R1 staining was detectable. In both cases it is probable, that the immunologic disease activity was extremely low, as shown by the barely detectable positivity for IgG by immunohistochemistry, the very few and small electron dense deposits in electron microscopy and the serological negativity for PLA2R1 antibody by both routine serological tests (immunofluorescence test and ELISA). We were able to identify these patients as PLA2R1-antibody positive only by very sensitive western blot analyses, which are not routinely available, but rather in scientific labs only. Therefore, in these two cases both immunohistochemical detection of the PLA2R1 antigen in the kidney and serological detection of PLA2R1-antibodies in the blood might have reached their sensitivity limits.
In 10% of PLA2R1-antibody-negative patients we also found second renal diseases, in addition to membranous nephropathy. Interestingly, the spectrum of these diseases was much broader compared to patients with PLA2R1-antibody positive membranous nephropathy. Whether in some of these cases both diseases were caused by common pathomechanisms remain unclear. We found some statistically significant differences in the histomorphological characteristics of PLA2R1-antibody-positive and PLA2R1-antibody-negative patients, however, considering the relatively low differences in the absolute values of all these characteristics, their suitability for a reliable differentiation of PLA2R1-associated from PLA2R1-antibody-negative membranous nephropathy is limited.
In conclusion, in this study we found a second renal diagnosis in the biopsy of 6% of patients with PLA2R1-antibody-positive membranous nephropathy and in 10% of patients with PLA2R1-antibody-negative membranous nephropathy, especially in patients with impaired renal function. Diabetic nephropathy, interstitial nephritis and IgA nephropathy were the diagnoses made in patients with PLA2R1-antibody-positive membranous nephropathy, while in PLA2R1-antibody-negative membranous nephropathy a broader range of additional renal diseases was observed. Our data suggest that in nephrotic PLA2R1-antibody-positive patients a more individualised approach to the decision on whether to perform a kidney biopsy is possible and considering the information provided by the biopsy this procedure should be performed in patients who do not have a high risk for complications.
References
Ehrenreich T, Churg J. Pathology of membranous nephropathy. Path Ann. 1968;3:145–86.
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21.
Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797–804.
Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant. 2013;28:1839–44.
Beck LH Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:543–1550.
Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1735–43.
Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25:1357–66.
Chunduri S, Whittier WL, Korbet SM. Adequacy and complication rates with 14- vs. 16-gauge automated needles in percutaneous renal biopsy of native kidneys. Semin Dial. 2015;28:E11–E14.
Corapi KM, Chen JL, Balk EM, Gordon CE. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60:62–73.
Hoxha E, Beck LH Jr, Wiech T, Tomas NM, Probst C, Mindorf S, et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol. 2017;28:520–31.
Hogan JJ, Mocanu M, Berns JS. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11:354–62.
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney inter., Suppl. 2012;2: 139–274.
Evans DJ, Macanovic M, Dunn MJ, Pusey CD. Membranous glomerulonephritis associated with follicular B-cell lymphoma and subepithelial deposition of IgG1-kappa paraprotein. Nephron Clin Pract. 2003;93:c112–118.
Acknowledgements
We thank Sonia Wulf, Sandra Freyberg and Eugen Kinzler for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This study was supported by a grant from the Deutsche Forschungsgemeinschaft as part of the Sonderforschungsbereich 1192 (project B1 to EH and RAKS, project C1 to RAKS and TW and project B6 to TW). EH is supported by the Else Kröner-Fresenius Stiftung.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wiech, T., Stahl, R.A.K. & Hoxha, E. Diagnostic role of renal biopsy in PLA2R1-antibody-positive patients with nephrotic syndrome. Mod Pathol 32, 1320–1328 (2019). https://doi.org/10.1038/s41379-019-0267-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0267-z
This article is cited by
-
Diagnostic value of renal biopsy in anti-phospholipase A2 receptor antibody-positive patients with proteinuria in China
Scientific Reports (2024)
-
Deep learning-based multi-model approach on electron microscopy image of renal biopsy classification
BMC Nephrology (2023)
-
The classical pathway triggers pathogenic complement activation in membranous nephropathy
Nature Communications (2023)
-
Anti-phospholipase A2 receptor antibody levels at diagnosis predicts outcome of TAC-based treatment for idiopathic membranous nephropathy patients
BMC Nephrology (2022)
-
Characterization of THSD7A-antibodies not binding to glomerular THSD7A in a patient with diabetes mellitus but no membranous nephropathy
Scientific Reports (2021)