Abstract
Angioimmunoblastic T-cell lymphoma is a peripheral T-cell lymphoma derived from follicular T-helper cells. High-throughput genomic sequencing studies have shown that angioimmunoblastic T-cell lymphoma carries frequent mutations in RHOAG17V and IDH2R172 genes. The clinico-pathological features of angioimmunoblastic T-cell lymphoma cases with RHOAG17V mutations have been addressed; however, similar studies for IDH2 mutated cases are lacking. Therefore, the aim of the present study was to evaluate the pathological features of angioimmunoblastic T-cell lymphoma with IDH2 mutations. In order to identify cases with IDH2 mutations, 50 cases previously diagnosed as angioimmunoblastic T-cell lymphoma were subjected to next-generation sequencing analysis using a custom panel covering four genes frequently mutated in angioimmunoblastic T-cell lymphoma including DNMT3A, TET2, IDH2 and RHOA. All cases were analyzed for PD1, ICOS, CXCL13, CD10, BCL6, CD21, CD23 and EBER in situ hybridization. Mutational analysis recognized three groups. Group 1: IDH2R172 mutations were identified in 20 cases (40%). All cases carried RHOAG17V mutations. Group 2: RHOAG17V mutations without IDH2R172 mutation were identified in 16 cases (32%), and Group 3: 14 cases (28%) without RHOAG17V or IDH2R172 mutations. Morphologically, angioimmunoblastic T-cell lymphoma cases with IDH2R172 mutations were characterized by the presence of medium to large clear cells (p = 0.00001), and a follicular T-helper phenotype with the particular feature of strong CD10 (p = 0.0268) and CXCL13 expression (p = 0.0346). Interestingly, TET2 mutations were identified in 32 of 33 (97%) cases with IDH2R172 and/or RHOAG17V mutations whereas only 55% of angioimmunoblastic T-cell lymphoma cases wild-type for these two genes carried TET2 mutations (p = 0.0022). In contrast, DNMT3A mutations were found in 48% of the cases and were equally distributed in the three groups. In conclusion, our results support the results of gene expression profiling studies suggesting that IDH2R172 mutations define a unique subgroup within angioimmunoblastic T-cell lymphoma with strong follicular T-helper-like phenotype and characteristic morphological features.
Similar content being viewed by others
Introduction
Angioimmunoblastic T-cell lymphoma is a peripheral T-cell lymphoma derived from follicular T-helper cells [1, 2]. The 2016 revised World Health Organization (WHO) classification of lymphomas recognizes, in addition to angioimmunoblastic T-cell lymphoma, two provisional lymphoma entities derived from follicular T-helper cells, namely follicular peripheral T-cell lymphoma and nodal peripheral T-cell lymphoma with a follicular T-helper phenotype [3, 4]. These lymphomas are not only derived from follicular T-helper cells but also share clinical and genetic features, as well as molecular signatures suggesting that they most probably represent a different spectrum of the same disease [5].
Angioimmunoblastic T-cell lymphoma is usually a disorder of older adults who present with advanced stage, with frequent evidence of systemic immune dysregulation, skin rash, generalized lymphadenopathy and aggressive clinical course [6, 7]. The pathological features are rather characteristic with partial or complete effacement of the lymph node architecture by small to medium-sized T cells accompanied by a polymorphic inflammatory cell infiltrate [4, 8, 9]. The presence of clear cells, arborizing high-endothelial venules and proliferation of follicular dendritic cells are variably observed. Another typical finding is presence of EBV-positive immunoblasts detectable in the majority of the cases that may rarely progress to a clonal B-cell proliferation and mask the underlying T-cell lymphoma. Angioimmunoblastic T-cell lymphoma is derived from mature CD4+ T cells with expression of several follicular T-helper markers including PD1, ICOS, CXCL13, CD10, BCL6, SAP or CCR5 [2, 9,10,11].
In the last years, high-throughput genomic sequencing technologies have shed new light on the mutational profile of angioimmunoblastic T-cell lymphoma, revealing recurrent somatic mutations in epigenetic regulator genes such as ten-eleven translocation 2 (TET2), DNA methyltransferase 3A (DNMT3A) [12, 13] and isocitrate dehydrogenase 2 (IDH2) [14], mutations in RHOA [15,16,17,18,19], as well as activating mutations of the T-cell receptor signaling including CD28 [20]. Of these mutations, only IDH2 p.R172 (IDH2R172) seems to be restricted to angioimmunoblastic T-cell lymphoma, whereas TET2, DNMT3A and RHOA mutations can be found in other follicular T-helper-derived T-cell lymphomas. RHOA p.Gly17Val (RHOAG17V) mutation has been identified in up to 70% of angioimmunoblastic T-cell lymphoma cases, and because of its essential role in multiple T-cell functions including polarization, migration and signaling through the T-cell receptor, it has been suggested to play a role in the pathogenesis of the disease [16,17,18,19]. Furthermore, three recent studies using mouse models have shown that RHOAG17V induces follicular T-helper specification, autoimmunity and promotes lymphomagenesis in the presence of TET2 mutations, indicating that this combination has a synergistic effect on oncogenesis [17, 21, 22]. Accordingly, two studies have examined the presence of RHOAG17V mutation, and its association with pathological features [23, 24]. Cases with RHOAG17V mutation seem to have a higher microvessel density, express a greater number of follicular T-helper markers and tend to have more follicular dendritic cell proliferation than cases with wild-type RHOA.
Although IDH2R172 mutations are restricted to angioimmunoblastic T-cell lymphoma and rarely nodal peripheral T-cell lymphoma with follicular T-helper phenotype within lymphoid neoplasias, they are identified only in approximately a third of the cases [14]. IDH2 belongs to the IDH family, which includes three enzymes involved in the oxidative decarboxylation of isocitrate to produce α-ketoglutarate [25]. IDH1 is located in the cytosol whereas IDH2 and IDH3 are located in the mitochondria. IDH2R172 mutations cause an enzymatic gain of function that results in the production and accumulation of the oncometabolite 2-hydroxyglutarate (2HG) leading to the inhibition of both histone lysine demethylases (KDMs) and the TET family of DNA hydroxylases [25]. These inhibitions alter the epigenetic control of progenitor cells inducing DNA and repressive histone hypermethylation of genes involved in T-cell receptor signaling and T-cell differentiation that most probably contribute to the lymphomagenesis of angioimmunoblastic T-cell lymphoma [26, 27]. IDH2R172 mutations seem to define a unique subgroup of angioimmunoblastic T-cell lymphoma cases with distinct follicular T-helper-like gene expression signature [27]. Therefore, the aim of this study was to analyze the pathological features of angioimmunoblastic T-cell lymphoma with IDH2R172 mutations.
Materials and methods
Patient samples
A total of 50 samples with the diagnosis of angioimmunoblastic T-cell lymphoma and available formalin-fixed paraffin-embedded tissue, diagnosed between 2009 and 2016, were retrieved from the files of the Institute of Pathology, University Hospital of Tübingen. All cases were comprehensively immunophenotyped, as part of the diagnostic work-up, and were classified following the recommendations of the 2016 revised World Health Organization (WHO) classification for tumors of hematopoietic and lymphoid tissues [4]. All cases were evaluated with standard stains (hematoxylin and eosin (H&E), PAS and Giemsa). Pathologic variables were evaluated by three pathologists (ES-L, FF, LQ-M), who were blinded to the mutational status. These included histologic pattern, as previously published by Attygalle et al. [9], degree of follicular dendritic cell meshwork proliferation, proliferation of high-endothelial venules, presence of clear cells, B-cell proliferation, EBV quantification and follicular T-helper phenotype. The study was approved by the local ethics committee (090/2016BO2).
Immunohistochemistry and EBV EBER in situ hybridization
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections on the Ventana Ultra automated staining System (Ventana Medical Systems, Tucson, AZ, USA) using Ventana reagents, according to the manufacturer’s protocol. All cases were stained with a panel of antibodies to confirm the TFH phenotype including PD1 (ready to use, Roche Diagnostics GmbH, Mannheim, Germany), ICOS (Zytomed Systems GmbH, Berlin, Germany), CXCL13 (R&D Systems, Inc. Minneapolis, MN, USA), BCL6 (Zytomed) and CD10 (Leica Biosystems/Novocastra, Wetzlar, Germany). Additionally, stains for the follicular dendritic cell markers CD23 (Leica/Novocastra) and CD21 (Zytomed) were performed. A specific antibody against the IDH2R172K (New East 26163, USA) mutation was also used and performed manually [26,27,28]. EBER in situ hybridization was done in all cases using oligonucleotides complementary to EBER transcripts in an automated stainer (Ventana Medical Systems).
B-cell clonality analysis
Polymerase chain reaction (PCR) amplifications for detecting monoclonal immunoglobulin heavy (IGH) and kappa (IGK) light chain gene rearrangements were performed according to the BIOMED-2 protocol as previously described [29].
Next-generation sequencing analysis
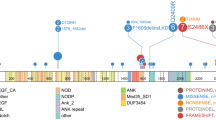
Angioimmunoblastic T-cell lymphoma cases were subjected to next-generation sequencing analysis using a custom panel covering four genes frequently mutated in this disease including DNMT3A, TET2, IDH2 and RHOA. Next-generation sequencing libraries were amplified using two primer pools of an Ion AmpliSeq custom panel. The panel covered 97.65% of all exons of DNMT3A and TET2 as well as hotspot regions of IDH2 (chr15:90631815–90631956, complete exon 4) and RHOA (chr3:49412940–49413020, complete exon 2). The custom panel was designed using the Ion AmpliSeq Designer from Thermo Fisher Scientific (version 6.0.4). Amplicon library preparation was performed using 10 ng of DNA for each primer pool, as advised by the manufacturer. Each DNA probe was mixed with AmpliSeq HiFi Mix and one of the two primer pools, containing 58 or 60 amplicons. For the PCR run the cycling conditions were as follows: Initial denaturation: 99 °C for 2 min, cycling: 24 cycles of 99 °C, 15 s and 60 °C, 4 min. After the end of the PCR reaction, primer end sequences were partially digested using FuPa reagent, followed by the ligation of barcoded sequencing adapters (Ion Xpress Barcode Adapters, Thermo Fisher Scientific). The final library was purified using AMPure XP magnetic beads (Beckman Coulter, Krefeld, Germany) and quantified using qPCR (Ion Library Quantitation Kit, Thermo Fisher Scientific) on a LightCycler 480 Instrument (Roche, Penzberg, Germany). Libraries were diluted to the same concentration (30–100 pM). Twelve libraries were then pooled and the pool diluted to a final concentration of 6 pM. The diluted library pool was processed to library amplification on Ion Sphere particles using the Ion OneTouch 2 instrument with the 200 bp chemistry. Unenriched libraries were quality controlled using Ion Sphere quality control measurement on a Qubit 3.0 Fluorometer (Thermo Fisher Scientific). After library enrichment (Ion OneTouch ES), the library was processed for sequencing using the Ion Torrent 200 bp sequencing chemistry and 12 barcoded libraries were sequenced onto a single 318 chip. Raw data analysis was performed using Ion Torrent Software Suite (Version 5.4). The mean coverage of the panel is 4848 reads and the mean coverage of the IDH2 variants represents 2253 reads. The reads were aligned to the human reference sequence build 38 (hg19) using the TMAP aligner implemented in the Torrent Suite software. Detection of single base pair variants and insertion−deletion polymorphisms (InDels) compared to the human 4 reference sequence was performed using Ion Torrent Variant Caller (5.4). Detection thresholds for SNPs and InDels were set at an allele frequency of 1%. Variants were annotated and filtered against the dbSNP and COSMIC databases using the Annotate variants single sample workflow of the Ion Reporter Software (version 4.2). Each variant was also inspected with the IGV software to exclude artifacts.
Statistical analysis
Angioimmunoblastic T-cell lymphoma cases with and without IDH2R172 mutations were compared using the Fisher exact test. All statistical tests were two-sided and statistical significance was concluded for values p < 0.05.
Results
Molecular analysis
In order to identify the angioimmunoblastic T-cell lymphoma cases with IDH2R172 mutations, mutation analysis was first performed. The 50 cases analyzed corresponded to 30 males and 20 female patients (M:F 1.5:1) with a median age of 71 years (range, 46−92 years). In 44 cases enough DNA quantity and quality was obtained (>200 bp) to perform the next-generation sequencing custom panel including four genes (TET2, DNMTA3, RHOA and IDH2) whereas in six cases only RHOA and IDH2 were investigated with single amplicons (see supplemental information). The results of the mutational analyses are summarized in Fig. 1. The mutational analysis revealed RHOAG17V mutations in 36/50 cases (72%), whereas IDH2R172 mutations were identified in 20/50 cases (40%). TET2 mutations were present in 38/44 cases (86%) with 23 cases showing more than one mutation. DNMT3A mutations were observed in 21/44 (48%) cases with only one case carrying more than one mutation. The median variant allelic frequency (VAF) for RHOAG17V and IDH2R172 mutations was 8% with a range of 1–31% (see Supplementary Tables 1−3). In cases where both genes were mutated the VAFs were very similar indicating that these two gene mutations occurred at similar times. In contrast, the median VAF for TET2 mutations was 19% (range 3–44%) taking into consideration only the TET2 mutation with the highest allelic frequency per case. The median VAF for DNMT3A was 10% (range 2–37%). In general, the allelic frequencies of TET2 and DNMT3A mutations were much higher than those of RHOAG17V and IDH2R172 indicating that these two mutations occurred first. However, in eight cases the allelic frequencies were the same in all genes, and in one case the allelic frequency of TET2 was lower than that of RHOAG17V mutation (Supplemental Tables 1−3). According to the mutational analysis, three groups were identified (Fig. 1). Group 1 was composed of 20 cases (40%) with IDH2R172 mutations (Supplemental Table 1). RHOAG17V mutations were identified in 100% of these cases, TET2 mutations in 19 cases (95%), whereas DNMT3A mutations were present in eight cases (40%). Group 2 was composed of 16 cases (32%) with RHOAG17V mutations without IDH2R172 mutation, all of which carried a TET2 mutation (13/13; 100%), whereas six cases (6/13; 46%) showed DNMT3A mutations (Supplemental Table 2). Group 3 was composed of 14 cases (28%) without RHOAG17V or IDH2R172 mutations. In this group TET2 and DNMT3A mutations were identified in 6 cases each of 11 (55%) analyzable cases (Supplemental Table 3). Taken together, TET2 mutations were identified in 32 of 33 (97%) cases with IDH2R172 and/or RHOAG17V mutations whereas only 55% of angioimmunoblastic T-cell lymphoma cases without these two mutations carried TET2 mutations. The difference was statistically significant (p = 0.0022).
Pathologic features of IDH2 R172 mutated cases
In all cases angioimmunoblastic T-cell lymphoma pattern, the presence of follicular dendritic cells, proliferation of high-endothelial venules, clear cells, B-cell proliferation, presence and quantity of EBER+ cells and the follicular T-helper phenotype (ICOS, PD1, CXCL13, CD10 and BCL6) were assessed. The pathologic features of Group 1 are summarized in Table 1. Pattern III characterized by total effacement of the nodal architecture without residual germinal centers was identified in 11 cases (55%). Pattern II characterized by the presence of multiple regressive “burnt-out” germinal centers was observed in eight cases (40%), whereas pattern I characterized by reactive, naked germinal centers and interfollicular expansion by lymphoma was observed in one case (5%). Several cases showed a combination of patterns, but the predominant pattern was selected. High-endothelial vascular proliferation as assessed with PAS stain was quite variable but present in all cases. The most prominent morphological feature in this group was the presence of medium to large clear cells identified in 16 cases (80%) (Fig. 2). The follicular dendritic cell meshwork proliferation was assessed with CD23 and CD21 and graded as negative, mild (+), moderate (++) and marked (+++) (Supplemental Fig. 1). Mild proliferation included cases that showed “beginning” expansion beyond the typical limits of a germinal center. Of the 11 cases with pattern III, only four cases showed marked follicular dendritic cell proliferation, three cases showed moderate proliferation and four cases showed no demonstrable follicular dendritic cell proliferation. Of the eight cases with pattern II, only one case showed moderate proliferation, four cases showed mild proliferation, and three cases showed no follicular dendritic cells proliferation. Altogether only 8/20 (40%) cases showed moderate to marked follicular dendritic cell proliferation characteristic of angioimmunoblastic T-cell lymphoma. B-cell proliferation was observed in 13 cases (65%), five of which showed striking plasma cell proliferation. IGH clonality analysis demonstrated a monoclonal population in three cases (23%), whereas 10 cases were polyclonal. The number of EBER+ cells varied from case to case; however, 11 cases (55%) showed numerous EBV-positive immunoblasts. In five cases only rare EBER+ cells were observed (25%), whereas four (20%) cases remained EBER negative.
Medium to large clear cells in angioimmunoblastic T-cell lymphoma with IDH2R172 mutation. a, b Examples of angioimmunoblastic T-cell lymphoma cases with pattern II. Note the clear cells surrounding the regressive “burnt-out” germinal centers. (a case 13 and b case 3) c−f Examples of angioimmunoblastic T-cell lymphoma cases with clear cells. The clear cells are scattered or in small groups surrounding high-endothelial venules (c case 7; d case 11; e case 6; f case 19) (H&E stain, ×200). H&E hematoxylin and eosin
All cases in this group showed a follicular T-helper phenotype with expression of the three markers ICOS, PD1 and CXCL13. Interestingly, CD10 was strongly and uniformly expressed in 15 (75%) cases including the case with pattern I of infiltration. This latter case showed a corona of clear cells around the reactive, naked germinal centers (Fig. 3a) that in addition to strong CD10 expression (Fig. 3b) was positive for PD1, ICOS and CXCL13 (Fig. 3c−e). In cases with pattern II the residual germinal centers were CD10 weakly positive, whereas the lymphoma cells were strongly positive (Fig. 4a, c). BCL6 was the least reliable marker with mostly weak expression in nine cases (53%). In addition, ten cases were stained with the IDH2 antibody that specifically recognizes the protein with the IDH2R172K mutation. IDH2R172K was identified in five cases (25%), all of which reacted positively with the antibody with good correlation with the allelic frequency identified by next-generation sequencing (Fig. 4b) and the staining pattern of other follicular T-helper markers (Fig. 4c−f). The case with the highest IDH2R172K allelic frequency (31%) showed a diffuse infiltration of rather large cells with clear cytoplasm (Fig. 5a). The IDH2 antibody was positive in the majority of the tumor cells (Fig. 5b), as well as other follicular T-helper markers (Fig. 5c−f); however, no follicular dendritic cell proliferation was demonstrated with CD21 and CD23 antibodies (Fig. 5g, h). The other five cases analyzed with different IDH2R172 mutations, as expected, remained negative. In summary, following the recommendations of the WHO classification, cases 17−20 with a diffuse infiltration of the lymph node (pattern III) and lack of follicular dendritic cell proliferation were classified as nodal peripheral T-cell lymphoma with follicular T-helper phenotype despite the presence of other morphological features of angioimmunoblastic T-cell lymphoma. The other 16 cases fulfilled all criteria of angioimmunoblastic T-cell lymphoma.
Angioimmunoblastic T-cell lymphoma case 8 with pattern I and IDH2R172 mutation. a H&E stain shows a naked hyperplastic germinal center surrounded by clear cells (original magnification, ×100). Insert: higher magnification demonstrates the cytology of the clear cells (original magnification, ×400). b CD10 stain reveals a weak staining in the hyperplastic germinal center whereas the clear cells surrounding the germinal center are homogeneously CD10 strongly positive (immunohistochemistry, ×50). c−e The clear cells are PD1 (c), ICOS (d) and CXCL13 (e) positive (immunohistochemistry, ×100). H&E hematoxylin and eosin
Angioimmunoblastic T-cell lymphoma case 4 with pattern II and IDH2R172 mutation. a Giemsa stain demonstrates a burnt-out germinal center surrounded by clear cells Insert: higher magnification to show the cytology of the clear cells (original magnification, ×200, insert, ×400). b The same case is stained with the specific antibody against the IDH2R172K mutation. Note how the distribution of the positive cells is similar to the distribution of the clear cells. Insert: higher magnification demonstrates the paranuclear cytoplasmic positivity characteristic of the antibody (original magnification, ×200, insert, ×400). The variant allelic frequency in this case was 17% by next-generation sequencing. c−f The clear cells are strongly positive for CD10 (c), PD1 (d), CXCL13 (e), and ICOS (f) (original magnification, ×200)
Nodal peripheral T-cell lymphoma with follicular T-helper phenotype case 18 with pattern III and IDH2R172 mutation. a H&E stain shows the diffuse pattern of infiltration with abundant clear cells and blood vessel proliferation. Insert: Higher magnification reveals the cytology of the large cells with atypical nuclei and some prominent nucleoli (original magnification, ×100, insert, ×400). b The specific antibody against the IDH2R172K mutation shows a diffuse expression in the tumor cells. Of note, this case has the highest variant allelic frequency; 31% by next-generation sequencing. Insert: Higher magnification reveals the perinuclear positivity (original magnification, ×200, insert, ×400). c−f The tumor cells are positive for CD10 (c), PD1 (d), ICOS (e), and CXCL13 (f) (original magnification, ×400). These stains highlight the broad clear cytoplasm of the tumor cells. g, h This case lacks follicular dendritic cell proliferation as demonstrated by CD21 (g) and CD23 (h) stains (original magnification, ×200). H&E hematoxylin and eosin
Comparison between angioimmunoblastic T-cell lymphoma cases with and without IDH2 R172 mutation
The comparison between angioimmunoblastic T-cell lymphoma cases with and without IDH2R172 mutation is summarized in Table 2. In total, 16 angioimmunoblastic T-cell lymphoma cases with IDH2R172 mutation were included for the statistical analysis. The most characteristic feature of IDH2R172 mutated cases was the presence of medium to large clear cells identified in the majority of the cases (p = 0.00001). These medium to large clear cells were not observed in the other groups where only in a minority of the cases mostly small clear cells were observed (Fig. 6). Although patterns I−II were more frequently observed in the IDH2R172 mutated group, the difference was not statistically significant. CD10 was more frequently observed in the IDH2R172 mutated group and the difference was statistically significant (p = 0.0268). However, the difference between the IDH2R172 and RHOAG17V-only group was not significant (p = 0.1489). CXCL13 was also more frequently expressed in the IDH2R172 mutated group (p = 0.0346). Follicular dendritic cell proliferation was observed in 12/16 cases (75%). As expected cases with patterns I and II showed few or no follicular dendritic cell proliferation. B-cell proliferation was frequently observed in all three groups; however, five of the six cases with plasma cell proliferation were in the IDH2R172 mutated group. Vascular proliferation was observed in all three groups. Cases with diffuse infiltration (pattern III), in general, revealed more vascular proliferation than cases with focal infiltration (patterns I or II), regardless of the mutational profile.
Angioimmunoblastic T-cell lymphoma case 50 with pattern III and no IDH2R172 or RHOAG17V mutations. a H&E stain shows the characteristic features of angioimmunoblastic T-cell lymphoma with abundant high-endothelial venules and a polymorphic infiltrate with some large activated cells. b CD20 highlights the presence of abundant activated B cells. c The B cells are also CD30 positive. Insert: EBER in situ hybridization reveals many EBV+ cells (original magnification, ×200). d−f. The tumor cells are PD1 (d), ICOS (f) and CXCL13 (f) positive. g CD23 stain reveals marked follicular dendritic cell proliferation characteristic of angioimmunoblastic T-cell lymphoma. H&E hematoxylin and eosin
Group 3 lacking both RHOA and IDH2 mutations showed follicular dendritic cell proliferation in all cases. High-endothelial vascular proliferation was prominent in all cases. These cases showed more often diffuse infiltration of the lymph nodes (pattern III; 77%), EBV-positive cells (64%) and expression of PD1, ICOS and CXCL13 but rarely expressed CD10 and BCL6 (Fig. 6a−g). Group 2 angioimmunoblastic T-cell lymphoma cases with RHOAG17V mutation showed features intermediate between Groups 1 and 3.
Discussion
In this study, we performed mutational analysis of 50 cases previously diagnosed as angioimmunoblastic T-cell lymphoma with a next-generation sequencing custom panel to identify cases with IDH2R172 mutations in order to analyze the associated pathological features. We confirmed the presence of IDH2R172 mutations in 40% of cases, which is slightly higher than the rate found in other studies [5, 14, 15]. All cases with IDH2R172 mutation carried RHOAG17V mutation and 95% of the cases TET2 mutations, whereas DNMT3A mutations were observed in 40% of the cases. We also demonstrated that angioimmunoblastic T-cell lymphoma cases with IDH2R172 mutations are characterized by the unique presence of medium to large clear cells (p = 0.00001) and strong CD10 (p = 0.0268) and CXCL13 expression (p = 0.0346). Interestingly, the association of TET2 with IDH2R172 and/or RHOAG17V mutation was identified in 97% of the cases, as opposed to cases without these two mutations, where in 55% of the cases TET2 mutations were found (p = 0.0022).
In contrast to previous studies [5, 13], a high co-occurrence of TET2 and IDH2R172 mutations was demonstrated by next-generation sequencing in this study (95%). Our results indicate that TET2 mutations are more frequent than previously reported in studies using Sanger sequencing. Accordingly, a recent study demonstrated TET2 mutations in 92% of angioimmunoblastic T-cell lymphoma cases (12/13 cases), when only PD1+ microdissected tumor cells were sequenced [30]. Additionally, three studies using whole exome or targeted deep sequencing detected TET2 mutation in 76%, 80% and 100% in angioimmunoblastic T-cell lymphoma cases, respectively [15, 18, 31]. Although in the study of Odejide et al., an overall frequency of 76% for TET2 mutations was reported, IDH2R172 mutated cases carried TET2 mutations in 88% of the cases. Another important observation in our study was that 23 of 38 (61%) TET2 mutated cases harbored two or three TET2 mutations suggesting a strong selective pressure for loss of TET2 function in angioimmunoblastic T-cell lymphoma. Of note, IDH2R172/TET2 double-mutant seems to be a specific phenomenon in angioimmunoblastic T-cell lymphoma, which rarely occurs in acute myeloid leukemia where these two mutations are mutually exclusive [14, 32]. The synergistic effect of IDH2R172/TET2 double-mutant was recently demonstrated by gene expression profiling, which showed that IDH2R172/TET2 double-mutant upregulates follicular T-helper-associated genes (IL21 and ICOS) and downregulate genes associated with TH1 (IL2, STAT1, CXCR13), TH2 (IL10RA) and TH17 (IL17RA) conferring a more follicular T-helper cell-like phenotype to the tumor cells [27]. We also confirmed previous reports that showed that in angioimmunoblastic T-cell lymphoma the allelic frequencies for TET2 and DNMT3A mutations are usually higher than that of IDH2R172 and RHOAG17V indicating that TET2 and DNMT3A mutations, in most cases, occur first [12, 33]. TET2 and DNMT3A mutations were first described in myeloid malignancies [32] but later on were detected in clonal hematopoiesis of “healthy” elderly individuals associated with increased risks to develop hematologic neoplasms [34, 35]. TET2 and DNMT3A mutations are believed to occur in a hematopoietic stem cell (HSC) that consequently acquires a proliferative advantage, but not sufficient to fully transform the cell. Furthermore, in angioimmunoblastic T-cell lymphoma, it has been shown that TET2 mutations are present both in tumor cells and in nontumor hematopoietic cells, whereas IDH2R172 mutation occurs only in follicular T-helper-like tumor cells [12]. Accordingly, using a specific antibody against the IDH2R172K mutant, identified in this study in 25% of the IDH2R172 mutated cases, we observed a good correlation of the IDH2R172K-positive cells with the expression of other follicular T-helper markers (CD10, PD1, ICOS and CXCL13) and with the IDH2R172 allelic frequency obtained with the next-generation sequencing analysis. These results suggest that the tumor cells in angioimmunoblastic T-cell lymphoma constitute a rather small portion of the infiltrate surrounded by a prominent reactive tumor microenvironment. Although the significance of early occurrence of TET2 and DNMT3A mutations in angioimmunoblastic T-cell lymphoma is not well understood, it is remarkable that group 3 in this study, comprising angioimmunoblastic T-cell lymphoma cases without IDH2R172 or RHOAG17V mutations, was the group with the lowest incidence of TET2 and DNMT3A mutations suggesting that these early mutations are important for the development of IDH2R172 and/or RHOAG17V mutations. Further studies are needed to understand the significance of the mutational landscape in angioimmunoblastic T-cell lymphoma.
Previous studies have shown that cases with RHOAG17V mutation have more frequently the morphological and immunophenotypic features of angioimmunoblastic T-cell lymphoma including significantly higher mean microvessel density, follicular dendritic cells proliferation and expression of several follicular T-helper markers when compared to wild-type cases [23, 24]. The morphological features of angioimmunoblastic T-cell lymphoma cases with IDH2R172 mutations have not been analyzed. Nevertheless, since recurrent IDH2R172 mutations usually are associated to RHOAG17V mutations, most probably some of the reported cases with typical features of angioimmunoblastic T-cell lymphoma carried also IDH2R172 mutations. The most striking morphological feature of IDH2R172 mutated cases was the presence of medium to large clear cells that were found only in this group. The presence of clear cells is considered a characteristic feature of angioimmunoblastic T-cell lymphoma that has been described in 41–50% of angioimmunoblastic T-cell lymphoma cases [9, 36]. Nevertheless, in previous studies the cytology of the clear cells (small vs. medium/large) was not discussed. Since IDH2 is located in the mitochondria, we speculated whether the presence of medium to large clear cells found only in the IDH2R172 mutated group might be secondary to mitochondrial changes; however, electron microscopic analysis performed in two IDH2R172 mutated cases (data not shown, supplemental Fig. 2) demonstrated the presence of normal mitochondria without evident explanation for the abundant clear cytoplasm observed in these tumor cells. Alternatively, since IDH2R172 mutations cause accumulation of the oncometabolite 2-hydroxyglutarate in the mitochondria, metabolic changes in the cell might be responsible for this peculiar morphology. The reason why these clear cells are present preferentially in cases with IDH2R172 mutations is unknown and warrants further studies.
The significant association demonstrated here between IDH2R172 mutation and the expression of follicular T-helper markers, especially CD10, supports the results of gene expression profiling studies suggesting that IDH2R172 mutations define a unique subgroup within angioimmunoblastic T-cell lymphoma with strong follicular T-helper-like phenotype [27]. Interestingly, in the original study by Attygalle et al. [9], CD10 expression was reported to be consistently expressed in the nests of large clear cells; and therefore, as evidence that in angioimmunoblastic T-cell lymphoma the neoplastic cells express CD10. We demonstrate here that this finding is characteristic of angioimmunoblastic T-cell lymphoma cases with IDH2R172 mutation. The reason why angioimmunoblastic T-cell lymphoma tumor cells express CD10 is not well understood; nevertheless, it has been suggested that CD10 expression may be an indicator of disturbed apoptotic cell death and immune deregulation [9]. More recently, it was demonstrated that CD10 expression identifies a subset of fully functional germinal center follicular T-helper cells with IL4 cytokine profile and with high capacity to sustain B-cell survival [37].
Another interesting feature associated with IDH2R172 mutation was the lack of follicular dendritic cell proliferation in some cases. This can be explained, in part, because there were eight cases with pattern II of infiltration that showed no or only mild follicular dendritic cell proliferation typical of pattern II. Nevertheless, there were 4 of 11 cases with diffuse infiltration of lymph nodes (pattern III) that did not show the characteristic follicular dendritic cell proliferation, as demonstrated by both CD21 and CD23 stains. Following the recommendations of the WHO, these cases were classified as nodal peripheral T-cell lymphoma, with follicular T-helper phenotype. However, the presence in three of the four cases of medium to large clear cells and strong CD10 and CXL13 expression are strong arguments in favor that these lesions, despite the lack of follicular dendritic cell proliferation, are biologically closely related. In fact, our results support the inclusion in the 2016 revised WHO classification of an “umbrella group” of nodal lymphomas with follicular T-helper phenotype including angioimmunoblastic T-cell lymphoma, follicular T-cell lymphoma and nodal peripheral T-cell lymphoma with follicular T-helper phenotype that most probably represent a spectrum of the same disease.
In conclusion, we demonstrate that angioimmunoblastic T-cell lymphoma cases with IDH2R172 mutations seem to represent a specific group with frequent co-occurrence of RHOAG17V and TET2 mutations and less frequent DNMT3A mutations, morphologically characterized by the presence of medium to large clear cells and follicular T-helper phenotype with strong CD10 and CXCL13 expression.
References
de Leval L, Gaulard P. Pathobiology and molecular profiling of peripheral T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2008:272–9.
de Leval L, Rickman DS, Thielen C, Reynies AD, Huang YL, Delsol G, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–63.
Swerdlow SH, Campo E, HarrisNL, Jaffe ES, Pileri SA, Stein H, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Dogan A, Gaulard P, Jaffe ES, Müller-Hermelink HK, de Leval L. Angioimmunoblastic T-cell lymphoma and other nodal lymphomas of T follicular helper cell origin. In: Swerdlow SH, editor. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon International Agency for Research on Cancer; Lyon, France, 2017. p. 407−12.
Dobay MP, Lemonnier F, Missiaglia E, Bastard C, Vallois D, Jais JP, et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica. 2017;102:e148–e51.
Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–30.
Federico M, Rudiger T, Bellei M, Nathwani BN, Luminari S, Coiffier B, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240–6.
Attygalle AD, Kyriakou C, Dupuis J, Grogg KL, Diss TC, Wotherspoon AC, et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression. Am J Surg Pathol. 2007;31:1077–88.
Attygalle A, Al-Jehani R, Diss TC, Munson P, Liu H, Du MQ, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627–33.
Dupuis J, Boye K, Martin N, Copie-Bergman C, Plonquet A, Fabiani B, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol. 2006;30:490–4.
Marafioti T, Paterson JC, Ballabio E, Chott A, Natkunam Y, Rodriguez-Justo M, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432–9.
Couronne L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T-cell lymphoma. N Engl J Med. 2012;366:95–6.
Lemonnier F, Couronne L, Parrens M, Jaïs JP, Travert M, Lamant L, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012;120:1466–9.
Cairns RA, Iqbal J, Lemonnier F, Kucuk C, de Leval L, Jais JP, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119:1901–3.
Odejide O, Weigert O, Lane AA, Toscano D, Lunning MA, Kopp N, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123:1293–6.
Palomero T, Couronne L, Khiabanian H, Kim MY, Ambesi-Impiombato A, Perez-Garcia A, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014;46:166–70.
Cortes JR, Ambesi-Impiombato A, Couronne L, Couronné L, Quinn SA, Kim CS, et al. RHOA G17V induces T follicular helper cell specification and promotes lymphomagenesis. Cancer Cell. 2018;33:259–73 e7.
Sakata-Yanagimoto M, Enami T, Yoshida K, Shiraishi Y, Ishii R, Miyake Y, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46:171–5.
Yoo HY, Sung MK, Lee SH, Kim S, Lee H, Park S, et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46:371–5.
Vallois D, Dobay MP, Morin RD, Lemonnier F, Missiaglia E, Juilland M, et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell-derived lymphomas. Blood. 2016;128:1490–502.
Ng SY, Brown L, Stevenson K, de Souza T, Aster JC, Louissaint A Jr, et al. RhoA G17V is sufficient to induce autoimmunity and promotes T-cell lymphomagenesis in mice. Blood. 2018;132:935–47.
Zang S, Li J, Yang H, Zeng H, Han W, Zhang J, et al. Mutations in 5-methylcytosine oxidase TET2 and RhoA cooperatively disrupt T cell homeostasis. J Clin Invest. 2017;127:2998–3012.
Nagao R, Kikuti YY, Carreras J, Kikuchi T, Miyaoka M, Matsushita H, et al. Clinicopathologic analysis of angioimmunoblastic T-cell lymphoma with or without RHOA G17V mutation using formalin-fixed paraffin-embedded sections. Am J Surg Pathol. 2016;40:1041–50.
Ondrejka SL, Grzywacz B, Bodo J, Makishima H, Polprasert C, Said JW, et al. Angioimmunoblastic T-cell lymphomas with the RHOA p.Gly17Val mutation have classic clinical and pathologic features. Am J Surg Pathol. 2016;40:335–41.
Yang H, Ye D, Guan KL, Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res. 2012;18:5562–71.
Lemonnier F, Cairns RA, Inoue S, Li WY, Dupuy A, Broutin S, et al. The IDH2 R172K mutation associated with angioimmunoblastic T-cell lymphoma produces 2HG in T cells and impacts lymphoid development. Proc Natl Acad Sci USA. 2016;113:15084–9.
Wang C, McKeithan TW, Gong Q, Zhang W, Bouska A, Rosenwald A, et al. IDH2R172 mutations define a unique subgroup of patients with angioimmunoblastic T-cell lymphoma. Blood. 2015;126:1741–52.
Dupuy A, Lemonnier F, Fataccioli V, Martin-Garcia N, Robe C, Pelletier R, et al. Multiple ways to detect IDH2 mutations in angioimmunoblastic T-cell lymphoma from immunohistochemistry to next-generation sequencing. J Mol Diagn. 2018;20:677–85.
Schmidt J, Gong S, Marafioti T, Mankel B, Gonzalez-Farre B, Balagué O, et al. Genome-wide analysis of pediatric-type follicular lymphoma reveals low genetic complexity and recurrent alterations of TNFRSF14 gene. Blood. 2016;128:1101–11.
Schwartz FH, Cai Q, Fellmann E, Hartmann S, Mäyränpää MI, Karjalainen-Lindsberg ML, et al. TET2 mutations in B cells of patients affected by angioimmunoblastic T-cell lymphoma. J Pathol. 2017;242:129–33.
Wang M, Zhang S, Chuang SS, Ashton-Key M, Ochoa E, Bolli N, et al. Angioimmunoblastic T cell lymphoma: novel molecular insights by mutation profiling. Oncotarget. 2017;8:17763–70.
Shih AH, Abdel-Wahab O, Patel JP, Levine RL. The role of mutations in epigenetic regulators in myeloid malignancies. Nat Rev Cancer. 2012;12:599–612.
Quivoron C, Couronne L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98.
Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–87.
Merchant SH, Amin MB, Viswanatha DS. Morphologic and immunophenotypic analysis of angioimmunoblastic T-cell lymphoma: emphasis on phenotypic aberrancies for early diagnosis. Am J Clin Pathol. 2006;126:29–38.
Ame-Thomas P, Hoeller S, Artchounin C, Misiak J, Braza MS, Jean R, et al. CD10 delineates a subset of human IL-4 producing follicular helper T cells involved in the survival of follicular lymphoma B cells. Blood. 2015;125:2381–5.
Acknowledgements
The authors would like to thank Claudia Hermann and Nadine Martin for their excellent technical assistance. IM is supported by a grant from the Deutsche Forschungsgemeinschaft (DFG, QU144/1–1 to LQ-M). This work was supported in part by a grant from the Leukemia and Lymphoma Society SCOR 7013-17 to PG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Steinhilber, J., Mederake, M., Bonzheim, I. et al. The pathological features of angioimmunoblastic T-cell lymphomas with IDH2R172 mutations. Mod Pathol 32, 1123–1134 (2019). https://doi.org/10.1038/s41379-019-0254-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0254-4
This article is cited by
-
Therapeutic challenges in peripheral T-cell lymphoma
Molecular Cancer (2024)
-
Diagnostic and prognostic molecular pathology of lymphoid malignancies
Virchows Archiv (2024)
-
Deciphering the spectrum of cutaneous lymphomas expressing TFH markers
Scientific Reports (2023)
-
Transgenic IDH2R172K and IDH2R140Q zebrafish models recapitulated features of human acute myeloid leukemia
Oncogene (2023)
-
Epigenetic regulation in hematopoiesis and its implications in the targeted therapy of hematologic malignancies
Signal Transduction and Targeted Therapy (2023)