Abstract
Endometrial endometrioid carcinoma is usually divided into three histological subgroups: grade 1 (G1), grade 2 (G2), and grade 3 (G3). Most cases of endometrial endometrioid carcinoma G1/2 have a favorable prognosis, although some can have unfavorable outcomes, especially when they involve elderly patients, with similarities to endometrioid carcinoma G3 and serous carcinoma. This retrospective study evaluated whether TP53 abnormalities in endometrial endometrioid carcinoma could be used to supplement the current grading system and improve its ability to predict clinical outcomes. Immunohistochemical expression of TP53 was analyzed using tissue microarrays from the surgically resected specimens of 475 patients with endometrial endometrioid carcinoma. Weak or moderate expression was defined as TP53-normal expression, while absent or strongly positive expression was defined as TP53-aberrant expression. The endometrial endometrioid carcinomas had originally been diagnosed as G1 (69%), G2 (18%), and G3 (13%). Univariate analyses revealed that TP53-aberrant expression was associated with poor survival in G1 and G2 cases, but not G3 cases. In addition, age (<60 years vs. ≥60 years) was correlated with TP53-aberrant expression in G1 cases (3% vs. 16%, p = 0.001), but not in G2 or G3 cases. Based on immunohistochemical TP53 expression, the endometrial endometrioid carcinomas were reclassified using a prognostic grading system as high-grade (G1 or G2 with TP53- aberrant expression, and G3 with TP53-normal or -aberrant expression) or low-grade (G1 or G2 with TP53-normal expression). The multivariate analyses revealed that the prognostic grading system (using histological grade and TP53 expression) could independently predict poor progression-free survival (hazard ratio: 2.91, p < 0.001) and overall survival (hazard ratio: 3.62, p < 0.001). Therefore, combining immunohistochemical TP53 expression with the traditional histological grading system for endometrial endometrioid carcinoma may help improve its ability to accurately predict the patient’s prognosis.
Similar content being viewed by others
Introduction
Endometrial endometrioid carcinoma is the most common histological type of uterine body cancer through the entire patient age spectrum. This tumor is usually divided into three histological groups based on architectural and cytological atypia/abnormality: grade 1 (G1), grade 2 (G2), and grade 3 (G3) [1]. Endometrial carcinoma has also been classified as type I or type II by Bokhman, based on clinical, endocrine, and epidemiological findings [2, 3]. Type I endometrial carcinoma comprises endometrioid carcinoma G1/2, which is characterized by estrogenic dependency and a better prognosis, with a histogenetic background of endometrial hyperplasia. In contrast, type II endometrial carcinoma is comprising serous carcinoma, clear cell carcinoma, and some endometrioid carcinoma G3 tumors, which are non-estrogenic and have a poorer prognosis, with endometrial atrophy as their histogenesis [2, 3]. However, some of the endometrioid carcinoma G1/2 cases have an unfavorable prognosis, especially when they involve elderly patients, as they exhibit a biological behavior that is similar to that of type II endometrial carcinoma [4]. Histology is insufficient to predict clinical course for endometrial endometrioid carcinoma, as there is substantial heterogeneity in the biological and molecular features [3].
Several molecular biomarkers for prognostic prediction in endometrial carcinoma such as TP53 (tumor protein 53) [5], CTNNB1 (encodes β-catenin) [5,6,7], and POLE (encodes a subunit of DNA polymerase epsilon that has a role in DNA replication) [8] mutations have been proposed, but simple and optimal methods have not yet been established. The TP53 tumor suppressor gene encodes a nuclear transcription factor that is involved in cell cycle arrest, apoptosis, and angiogenesis inhibition [9, 10]. It is the most commonly mutated tumor suppressor gene in many malignancies [9] and immunohistochemistry is an effective technique for identifying mutations [11, 12]. The TP53 protein is an immunohistochemical marker that is useful for diagnosing both endometrial and ovarian serous carcinomas [13, 14], with TP53 abnormalities detected in ~12% of endometrial endometrioid carcinoma G1 tumors, 25% of G2 tumors, and 42% of G3 tumors [15]. Several studies have indicated that immunohistochemical TP53 overexpression is linked to endometrial endometrioid carcinoma that is biologically aggressive and has high-grade potential [14,15,16,17,18,19,20]. However, there is no large cohort study that has evaluated TP53 abnormalities in endometrial endometrioid carcinoma and their relationships with patient outcomes, especially based on histological grade, cancer stage, and patient age.
This retrospective study evaluated whether abnormal TP53 expression in endometrial endometrioid carcinoma could help supplement the current grading system. Thus, we examined the clinical relationships of TP53 immunohistochemical expression with histological grade, cancer stage, and patient age. The results indicate that some seemingly low-grade endometrioid carcinoma tumors may actually exhibit higher grade behavior, which might support the use of additional treatments, such as adjuvant chemotherapy or lymph node dissection.
Materials and methods
Patients and samples
Patients’ electronic medical records from the Saitama Medical University International Medical Center were reviewed from 2007 to 2017. The retrospective protocol was approved by the institutional review board (13–165), and all methods were performed in accordance with the 1975 Declaration of Helsinki. A total of 475 patients with histologically confirmed endometrial endometrioid carcinoma were included in this study. The patients’ records were reviewed to obtain data regarding age, body mass index, International Federation of Obstetrics and Gynecology (FIGO) 2008 stage [21], histological grade [1], lymphovascular invasion, myometrium invasion depth, therapeutic methods, recurrence, progression-free survival, and overall survival.
Immunohistochemical staining
Immunohistochemistry was performed to evaluate TP53 expression in tissue microarrays (KIN-2; AZUMAYA, Tokyo, Japan). Two cylindrical cores (diameter: 3.0 mm) were obtained from each paraffin-embedded tissue block, which corresponded to the representative histological findings, and were inserted into a recipient block to create the tissue microarray blocks. The tissue microarray blocks were cut into serial 4-μm sections, which were automatically analyzed using the VENTANA BenchMark XT system (Ventana Medical Systems, Inc., AZ, USA) according to the manufacturer’s protocol. The primary antibodies were monoclonal mouse antibodies targeting TP53 (dilution: 1:50, clone DO-7; Dako, Kyoto, Japan). Tissue microarray sections were deparaffinized and pretreated using Cell Conditioning 1 (Ventana Medical Systems, Inc., AZ, USA) for 90 min at pH 9.0 to achieve antigen retrieval, which was followed by inactivation of endogenous peroxidases and incubation with the primary anti-TP53 antibody at 37 °C for 32 min. Antigen-antibody reactions were visualized using the Ultraview DAB Detection Kit (Ventana Medical Systems, Inc., AZ, USA).
Interpretation of the immunohistochemical results
The evaluations of immunohistochemical TP53 expression were performed by one pathologist (Masanori Yasuda) and one physician (Mitsutake Yano), who specialize in gynecological oncology and were blinded to the patients’ clinicopathological parameters (Fig. 1). The results were scored as completely negative (0%), weakly positive (1–25%), moderately positive (26–80%), and strongly positive (>80%). For the present study, in order to identify differences in progression-free survival and overall survival, the results were classified as TP53-normal expression (weakly and moderately positive) or TP53-aberrant expression (completely negative or strongly positive).
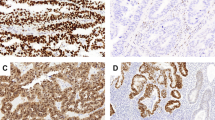
Staining with hematoxylin and eosin and TP53 immunohistochemistry. Results for endometrial endometrioid carcinoma G1 with TP53-normal expression (a: HE, b: TP53), G1 with TP53-aberrant expression (c: HE, d: TP53), G2 with TP53-normal expression (e: HE, f: TP53), G2 with TP53-aberrant expression (g: HE, h: TP53), G3 with TP53-normal expression (i: HE, j: TP53), and G3 with TP53-aberrant expression (k: HE, l: TP53). HE hematoxylin and eosin
Statistical analysis
The patients’ clinicopathological and immunohistochemical characteristics were compared using Pearson’s chi-square test or Fisher’s exact test, as appropriate. Survival curves were compared using the Kaplan–Meier method and log-rank test. Univariable and multivariable survival analyses were performed using the Cox proportional hazards model. All analyses were performed using IBM SPSS software (version 24.0; IBM Corp., Armonk, NY, USA), and differences were considered statistically significant at p-values of <0.05.
Results
Patient characteristics
Table 1 shows the patients’ characteristics, with the cases being classified as endometrial endometrioid carcinoma G1 (327 cases, 69%), G2 (88 cases, 18%), or G3 (60 cases, 13%). Based on the immunohistochemical expression of TP53, the patients were classified as having TP53-aberrant expression (80 cases, 17%) and TP53-normal expression (395 cases, 83%). The presence of TP53-aberrant expression was closely associated with a high FIGO stage (p < 0.001), lymphovascular invasion (p < 0.001), and deep myometrial invasion (p < 0.001).
Impact of TP53 status on clinical outcomes
The Kaplan–Meier analyses (Fig. 2a, b) revealed that TP53-aberrant expression was associated with poorer progression-free survival and poorer overall survival than TP53-normal expression in G1 cases (p < 0.001 and p < 0.001, respectively) and G2 cases (p = 0.010 and p = 0.047, respectively), but not in G3 cases (p = 0.616 and p = 0.344, respectively) (Fig. 2c). Furthermore, patients with endometrioid carcinoma G3 had poorer progression-free survival (Fig. 2d, e) and poorer overall survival than patients with endometrioid carcinoma G1/2 in all cases (p < 0.001 and p < 0.001, respectively) and in the TP53-normal expression group (p < 0.001 and p < 0.001, respectively), but not in the TP53-aberrant expression group (p = 0.640 and p = 0.359, respectively) (Fig. 2f). The presence of TP53-aberrant expression was associated with poor progression-free survival and overall survival regardless of the histological grade (Fig. 2f). In addition, poor outcomes were observed in the groups of endometrioid carcinoma G1/2 with TP53-aberrant expression, as well as endometrioid carcinoma G3 regardless of FIGO stage (Fig. 2g, i).
Kaplan–Meier survival analysis. The relationship between TP53 status and progression-free survival for endometrial endometrioid carcinoma G1 a, G2 b, and G3 c. The correlation between histological grade and progression-free survival for all patients d, with TP53-normal expression e, and with TP53-aberrant expression f. The correlation between TP53 status and progression-free survival for all-stage g, early-stage (I/II) h, and advanced-stage (III/IV) endometrial endometrioid carcinoma i. Asterisks indicate the p-values for comparing G1/2 with TP53-normal or -aberrant expression, and daggers indicate the p-values for comparing G1/2 with TP53-aberrant expression to G3. p-values were calculated using the log-rank test
Correlation between age and TP53 status
Figure 3a shows the correlation between age and the frequency of TP53-aberrant expression. In endometrial endometrioid carcinoma G1, TP53-aberrant expression was more frequent in patients who were ≥60 years old, although it was also more frequent in all endometrioid carcinoma G2/3 cases regardless of age. There was a significant correlation between age (<60 years vs. ≥60 years) and TP53-aberrant expression in G1 cases (3 vs. 16%, p = 0.001), but not in G2 cases (15 vs. 28%, p = 0.126) or G3 cases (58 vs. 47%, p = 0.440) (Fig. 3b). Figure 3c shows the stratification of endometrial endometrioid carcinoma according to FIGO grade, patient age, and immunohistochemical TP53 expression. Younger (<60 years) G1 and all G3 patients do not require TP53 immunohistochemistry due to low frequency of TP53-aberrant expression (Fig. 3a, b) and poor prognosis independent of TP53 status (Fig. 2c), respectively. Elderly (≥60 years) G1 and all G2 patients require TP53 immunohistochemistry, because their prognosis strongly depends on TP53 status (Fig. 2a, b).
Stratification according to age, histological grade, and TP53 status. a The ratio (%) of TP53 status for each 5-year interval in each histological grade and b the ratio (%) of TP53 status based on a cutoff value of 60 years. c Stratification according to histological grade, age, and TP53 status. d Schema of the prognostic grading system. G1/2 with TP53-normal expression was reclassified as low-grade endometrial endometrioid carcinoma, and G1/2 with TP53-aberrant expression plus G3 (TP53-normal or -aberrant expression) were reclassified as high-grade endometrial endometrioid carcinoma
Combination of the current grading system with TP53 status
Based on the prognosis (Fig. 2g–i), we reclassified G1/2 cases with TP53-normal expression as low-grade endometrial endometrioid carcinoma, and G1/2 cases with TP53-aberrant expression or all G3 cases (with TP53-normal or -aberrant expression) as high-grade endometrial endometrioid carcinoma (Fig. 3d). The Kaplan–Meier analysis revealed that, unlike the conventional histological grade (Fig. 4a, c), the prognostic grading system could predict poor outcomes regardless of stage (Fig. 4d, i). In FIGO stage III/IV cases, the 5-year rates of progression-free survival were 40% for G1/2, 33% for G3 (Fig. 4c), 46% for low-grade endometrial endometrioid carcinoma, and 28% for high-grade endometrial endometrioid carcinoma (Fig. 4f). In FIGO stage I/II cases, patients with low-grade endometrial endometrioid carcinoma had an extremely good 5-year prognosis (progression-free survival: 96%, overall survival: 97%) (Fig. 4e, h). In the multivariate survival analyses, which included all FIGO stages and histological grades (Table 2), the independent prognostic factors were the prognostic grading system (progression-free survival, hazard ratio: 2.91, 95% confidence interval: 1.85–4.56, p < 0.001; overall survival, hazard ratio: 3.62, 95% confidence interval: 2.21–5.94, p < 0.001) and FIGO staging (progression-free survival, hazard ratio: 3.06, 95% confidence interval: 2.41–3.89, p < 0.001; overall survival, hazard ratio: 2.83, 95% confidence interval: 2.19–3.66, p < 0.001). The prognostic grading system was also the independent prognostic factor (progression-free survival, hazard ratio: 5.66, 95% confidence interval: 2.64–12.1, p < 0.001; overall survival, hazard ratio: 6.34, 95% confidence interval: 2.78–14.5, p < 0.001) in the multivariate survival analyses for early-stage (I/II) endometrial endometrioid carcinoma.
Kaplan–Meier survival analysis. The correlations between histological grade and progression-free survival for all-stage a, early-stage (I/II) b, and advanced-stage (III/IV) endometrial endometrioid carcinoma c. The correlation between the prognostic grading system and progression-free survival for all-stage d, early-stage (I/II) e, and advanced-stage (III/IV) endometrial endometrioid carcinoma f. The correlation between the prognostic grading system and overall survival for all-stage g, early-stage (I/II) h, and advanced-stage (III/IV) endometrial endometrioid carcinoma i. p-values: log-rank test
Discussion
Molecular biomarkers are routinely used in diagnosis and clinical management in several cancers. The National Comprehensive Cancer Network Guidelines for breast cancer (version 3.2018) incorporate ER/PR/HER2 data into a provocative Pathologic Prognostic Stage scheme that is a better prognostic indicator than simple TNM staging [22]. The World Health Organization strongly supports molecular testing [Isocitrate dehydrogenase (IDH) mutations, 1p/19q codeletion, and so on] to better stratify patient prognosis for certain types of glioma [23]. Oropharyngeal squamous cell carcinomas have also been classified by p16/human papilloma virus status [24]. There are many biomarkers directly linked to treatment, such as EGFR/ALK/PD-L1 for lung cancer [25, 26] and BRAF for melanoma [27]. However, there are currently no predictive and/or prognostic molecular markers that are used in the routine clinical management of endometrial endometrioid carcinoma. The Cancer Genome Atlas Research Network has reported four genomic classes in endometrial carcinoma: POLE (a novel ultramutated group harboring POLE), microsatellite-unstable high or low copy-number tumors, copy-number aberrations, and microsatellite instability [8]. However, this classification requires expensive and detailed genetic analysis that may preclude routine use. CTNNB1 [5,6,7] and/or TP53 [5, 19] mutations also identify low grade endometrial endometrioid carcinoma patients at increased risk of recurrence. Immunohistochemical abnormality of β-catenin (encoded by CTNNB1) has high specificity in distinguishing CTNNB1 mutant from wild type, but sensitivity was lower [28]. β-catenin expressions cannot be used to determine prognosis in endometrial carcinoma patients [19, 29]. Interestingly, CTNNB1 mutation is more common in younger endometrial endometrioid carcinoma patients and rarely occurs simultaneously with TP53 mutation [5, 7]. These data suggest that detection of CTNNB1 mutation can be an auxiliary method of diagnosis in young endometrial endometrioid carcinoma patients with normal TP53. In comparison with β-catenin, TP53 immunohistochemistry has high specificity and sensitivity distinguishing TP53 mutant from wild type [12]. Of course, this is by no means 100%, because posttranslational modifications could have an effect on protein degradation or nuclear export/import, genetic or epigenetic changes of the protein degradation machinery, or even intronic gene mutations [12, 28]. However, the present study supports the prognostic utility of TP53 immunohistochemical patterns (not necessarily gene mutation) in endometrial endometrioid carcinoma, which agrees with the findings of several studies that support TP53 abnormalities being associated with biological aggressiveness and a poor prognosis [14,15,16,17,18,19,20].
We confirmed that TP53 immunohistochemical status is useful information, especially for all patients with endometrial endometrioid carcinoma G2 and elderly patients with G1, and G1/2 with TP53-aberrant expression exhibited a similar prognosis to G3. Garg et al. [14] and Hu et al. [30] have also indicated that TP53 immunohistochemical staining of morphologically ambiguous endometrial carcinoma can help guide prognostication and therapeutic decision making. Han et al. [31] have reported that ancillary techniques including TP53 immunohistochemistry improved the inter- and intra-observer agreement regarding the histological type, relative to the use of morphology alone. Given the potential limitations of using the current histological grading system for endometrial endometrioid carcinoma, routine application of TP53 immunohistochemical staining is recommended for cases of endometrial endometrioid carcinoma G1/2. Because endometrial endometrioid carcinoma G1/2 with TP53-aberrant expression had prognostic similarity with G3, both should receive similar clinical management. Additionally, given that endometrial endometrioid carcinoma G1/2 with TP53-normal expression had an extremely good prognosis in the early-stage (FIGO stages I/II), adjuvant therapy or lymph node dissection might be unnecessary in those cases. Immunohistochemical staining for TP53 is a simple and convenient technique that can be routinely performed at most facilities, which may help improve prognostication and diagnostic reproducibility for endometrial endometrioid carcinoma. Thus, based on our data, we believe that clinical management should be designed differently for high-grade (G1/2 with TP53-aberrant expression and all G3 cases) and low-grade endometrial endometrioid carcinoma (G1/2 with TP53-normal expression) (Fig. 3c, d), especially as our prognostic grading system could independently predict the patients’ prognoses.
The present study has several limitations, despite its relatively large sample size. First, the study only included Japanese patients, and further verification in other populations is necessary, given the racial disparities in the molecular subtypes of endometrial carcinoma [32]. Second, the use of tissue microarrays carries a risk of false-negative results, which may be related to tissue fixation and tumor heterogeneity. However, our experience with routine use of small biopsy specimens suggests that there is little or no discrepancy in TP53 expression between the biopsy specimen and the surgically resected material. Therefore, even the pre-operative biopsy specimen can likely be used to accurately examine TP53 expression.
In conclusion, combining immunohistochemical staining for TP53 expression with the traditional histological grading system for endometrial endometrioid carcinoma may improve ability to accurately diagnose endometrial endometrioid carcinoma and predict the subsequent prognosis.
References
Zaino RJ, Kurman RJ, Diana KL, et al. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer. 1995;75:81–6.
Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17.
Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15:e268–e278.
Ogane N, Hori SI, Yano M, et al. Preponderance of endometrial carcinoma in elderly patients. Mol Clin Oncol. 2018;9:269–73.
Kurnit KC, Kim GN, Fellman BM, et al. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol. 2017;30:1032–41.
Myers A, Barry WT, Hirsch MS, et al. beta-Catenin mutations in recurrent FIGO IA grade I endometrioid endometrial cancers. Gynecol Oncol. 2014;134:426–7.
Liu Y, Patel L, Mills GB, et al. Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J Natl Cancer Inst. 2014;106:dju245.
Cancer Genome Atlas Research Network, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73.
Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–6.
Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10.
Iggo R, Gatter K, Bartek J, et al. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990;335:675–9.
Kobel M, Piskorz AM, Lee S, et al. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J Pathol Clin Res. 2016;2:247–58.
Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–53.
Garg K, Leitao MM Jr, Wynveen CA, et al. p53 overexpression in morphologically ambiguous endometrial carcinomas correlates with adverse clinical outcomes. Mod Pathol. 2010;23:80–92.
Ito K, Watanabe K, Nasim S, et al. Prognostic significance of p53 overexpression in endometrial cancer. Cancer Res. 1994;54:4667–70.
Dupont J, Wang X, Marshall DS, et al. Wilms Tumor Gene (WT1) and p53 expression in endometrial carcinomas: a study of 130 cases using a tissue microarray. Gynecol Oncol. 2004;94:449–55.
Jongen VH, Briet JM, de Jong RA, et al. Aromatase, cyclooxygenase 2, HER-2/neu, and p53 as prognostic factors in endometrioid endometrial cancer. Int J Gynecol Cancer. 2009;19:670–6.
Lee EJ, Kim TJ, Kim DS, et al. p53 alteration independently predicts poor outcomes in patients with endometrial cancer: a clinicopathologic study of 131 cases and literature review. Gynecol Oncol. 2010;116:533–8.
Nout RA, Bosse T, Creutzberg CL, et al. Improved risk assessment of endometrial cancer by combined analysis of MSI, PI3K-AKT, Wnt/beta-catenin and P53 pathway activation. Gynecol Oncol. 2012;126:466–73.
Urabe R, Hachisuga T, Kurita T, et al. Prognostic significance of overexpression of p53 in uterine endometrioid adenocarcinomas with an analysis of nuclear grade. J Obstet Gynaecol Res. 2014;40:812–9.
Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109.
National Comprehensive Cancer Network. Development and update of the NCCN guidelines. [cited 1 January 2019]. Available from https://www.nccn.org/professionals/development.aspx.
Louis DN, von Deimling A, Cavenee WK. Diffuse astrocytic and oligodendroglial tumours. In: Louis DN, Ohgaki H, Wiestler OD, et al., eds. WHO Classification of Tumours of the Central Nervous System. WHO Classification of Tumours, Revised 4th edition. Vol 1. France: International Agency for Research on Cancer; 2016. p 16–7.
Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39.
Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. 2017;548:234–8.
Kim G, Kurnit KC, Djordjevic B, et al. Nuclear beta-catenin localization and mutation of the CTNNB1 gene: a context-dependent association. Mod Pathol. 2018;31:1553–9.
Sal V, Demirkiran F, Erenel H, et al. Expression of PTEN and beta-Catenin and their relationship with clinicopathological and prognostic factors in endometrioid type endometrial cancer. Int J Gynecol Cancer. 2016;26:512–20.
Hu S, Hinson JL, Matnani R, et al. Are the uterine serous carcinomas underdiagnosed? Histomorphologic and immunohistochemical correlates and clinical follow up in high-grade endometrial carcinomas initially diagnosed as high-grade endometrioid carcinoma. Mod Pathol. 2018;31:358–64.
Han G, Sidhu D, Duggan MA, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol. 2013;26:1594–604.
Dubil EA, Tian C, Wang G, et al. Racial disparities in molecular subtypes of endometrial cancer. Gynecol Oncol. 2018;149:106–16.
Acknowledgements
We thank Kouichi Kamada, Yusuke Hosonuma, Satoshi Kanno, Nobuyuki Suzuki, Yasuo Kamakura, and Akemi Miyata (Department of Pathology, Saitama Medical University International Medical Center) for their technical support. We also thank Editage (www.editage.jp) for English language editing.
Funding
This study was funded by the Hidaka Research Projects from Saitama Medical University (30-D-1-08) and Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (18K06997).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yano, M., Ito, K., Yabuno, A. et al. Impact of TP53 immunohistochemistry on the histological grading system for endometrial endometrioid carcinoma. Mod Pathol 32, 1023–1031 (2019). https://doi.org/10.1038/s41379-019-0220-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0220-1
This article is cited by
-
Construction of the novel immune risk scoring system related to CD8+ T cells in uterine corpus endometrial carcinoma
Cancer Cell International (2023)
-
Vulvar neuroendocrine carcinoma that is independent of merkel cell polyomavirus and human papillomavirus suggests endometrial cancer recurrence: a case report
BMC Endocrine Disorders (2022)