Abstract
We describe the histology and the frequency of a histologic entity that we term “hyaline fibrous involution”, which is characterized by symmetric and regular deposition of basal lamina-like periacinar hyaline material in association with atrophic epithelium, in breast samples from patients with either benign breast disease or germline BRCA mutation. Women with germline BRCA mutation (n = 93) who underwent prophylactic mastectomy (BRCA group) were compared to an age-matched sample of women who underwent biopsy for benign breast disease (n = 93). Median age was 45 years (range, 25–72 years). A single H&E section of each subject’s benign breast tissue was reviewed. The total number of terminal duct lobular units and the number of terminal duct lobular units with hyaline fibrous involution were recorded for each case. The presence of any hyaline fibrous involution lobules and the within-sample proportion of hyaline fibrous involution lobules relative to total lobules were compared between groups. Presence of any hyaline fibrous involution was significantly more frequent in the BRCA group compared to the benign breast disease group, 47% vs. 15% (p < 0.0001, adjusted for total lobules). In women with any hyaline fibrous involution lobules, these unusual lobules were similarly rare in both groups, with median proportion of hyaline fibrous involution-positive lobules relative to all lobules of 0.03 in BRCA specimens (n = 44) and 0.03 in the benign breast disease group (n = 14). Within the BRCA group, frequency of any hyaline fibrous involution present was significantly higher in the perimenopausal age group (45–55 years: 63%) compared to other age groups (<45 years, 44%; >55 years, 15%; p = 0.05 and p = 0.02, respectively). Increased presence of hyaline fibrous involution in the setting of BRCA mutation suggests that it may represent a pathologic entity, possibly reflecting abnormal involution or an abnormal response to DNA damage.
Similar content being viewed by others
Introduction
Lobular involution is an age associated physiological process in which terminal duct lobular units undergo progressive loss of cell populations over the course of decades, leading eventually to atrophy [1]. Involution results in terminal duct lobular units with fewer numbers of small acini that have flattened and inconspicuous epithelium along with loss of intralobular stroma [1]. There is evidence that delayed or interrupted involution is associated with increased risk for breast cancer development in women with benign breast disease [2, 3].
BRCA genes act as tumor suppressor genes and mediate double-stranded DNA break repair [4,5,6]. Pathogenic germline mutations in BRCA1 and BRCA2 are associated with a markedly increased susceptibility to breast carcinomas: female carriers of mutations in either gene have a lifetime risk of breast cancer of at least 50% [4, 5, 7]. Although the pathological features of breast cancers arising in the setting of BRCA germline mutations have been well-described, little is known about premalignant or benign alterations in the breast tissue of these women. Specifically, terminal duct lobular unit involution has not been studied.
This study describes a novel terminal duct lobular unit alteration that is characterized by periacinar deposition of basal lamina-like hyaline material in association with atrophic-appearing epithelium. This lobular alteration is similar to age-related lobular involution in terms of acinar loss, but different in the sense that it shows expansion of intralobular extracellular matrix by what appears to be pathological fibrosis. We have termed this lobular alteration ‘hyaline fibrous involution’ because of the hyalinized fibrosis in lobules demonstrating decreased diameter relative to (preinvolutional) normal lobules. We hypothesize that hyaline fibrous involution may reflect a pathological form of terminal duct lobular unit involution. The present study was undertaken to evaluate the possible association of hyaline fibrous involution with BRCA mutation status.
Materials and methods
Study population
Following approval by the Institutional Review Board of the Mayo Clinic, archival H and E stained sections were obtained from the Mayo Clinic Tissue Registry, including 93 women with germline deleterious BRCA mutations who underwent prophylactic mastectomies (1993–2012). A comparison group of 93 age-matched women was obtained from the Mayo Clinic Benign Breast Disease Cohort, which is a large, well-described cohort of ~13,538 women who underwent breast biopsy with benign findings from 1967–2001, including radiologically guided core needle biopsies starting in 1994 [8]. In this group, the BRCA mutation status is unknown. The benign breast disease samples for this study were restricted to the 1982–2001 portion of the benign breast disease cohort to make the tissue age more similar to that of the BRCA samples. A separate normal comparison group of 86 age-matched women who underwent reduction mammoplasty at Mayo Clinic Rochester in 1995–2001 was collected from Mayo Clinic files and archival H and E stained sections were obtained from the Mayo Clinic Tissue Registry.
Histologic review
For each subject, we reviewed all available slides and chose a representative one with the highest number of terminal duct lobular units. The selected representative benign tissue sections were examined to enumerate the total number of terminal duct lobular unit and the total number of terminal duct lobular unit with hyaline fibrous involution. Hyaline fibrous involution was defined as periacinar ring-like, regular deposition of basal lamina-like hyaline material in association with atrophic epithelium (Figs. 1 and 2). If a terminal duct lobular unit showed any hyaline fibrous involution regardless of the extent of involvement, it was called a “hyaline fibrous involution-positive lobule.” Hyaline fibrous involution-positive lobules were further categorized as diffusely or focally involved. A terminal duct lobular unit involved by hyaline fibrous involution in ≥50% of the total lobule area was designated as a “hyaline fibrous involution-diffuse lobule” (Fig. 2b), whereas hyaline fibrous involution in <50% of the lobule area was designated as a “hyaline fibrous involution-focal lobule” (Fig. 2d). The total numbers of terminal duct lobular units present on the slide, and the numbers of hyaline fibrous involution-diffuse lobules and hyaline fibrous involution-focal lobules, were evaluated and recorded separately in each case.
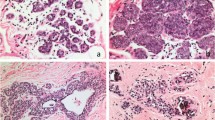
Comparison between age-related lobular involution and hyaline fibrous involution. a Non-involuted breast parenchyma of a premenopausal woman. b, c Age-related lobular involution characterized by terminal duct lobular units with fewer numbers of small acini that have flattened and inconspicuous epithelium along with loss of intralobular stroma. d–f Hyaline fibrous involution characterized by symmetric and regular, periacinar ring-like deposition of basal lamina-like hyaline material in association with atrophic epithelium
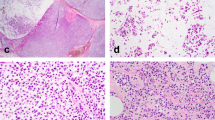
Photomicrographs of hyaline fibrous involution. a Low-magnification of breast parenchyma with hyaline fibrous involution. b A terminal duct lobular unit involved by hyaline fibrous involution in ≥50% of the total lobule area was designated as a “hyaline fibrous involution-diffuse lobule”. c High magnification of the hyaline fibrous involution seen in b. d A terminal duct lobular unit involved by hyaline fibrous involution in <50% of the total lobule area was designated as a “hyaline fibrous involution -focal lobule”. e, f A terminal duct lobular unit was diffusely involved by hyaline fibrous involution, with symmetric and regular, periacinar ring-like deposition of basal lamina-like hyaline material (e), which was stained blue in Masson-trichrome stain, suggestive of collagen type I (f)
Associations with clinical and pathologic features and follow-up
Associations of hyaline fibrous involution lobules with patient age and histologic age-related involution (“none” vs. “partial” vs. “complete”) were evaluated. Age-related involution was classified as none if <25% of normal lobules were involuted, as partial if 25–75% were involuted, and complete if >75% were involuted. Follow-up for breast cancer events in the benign breast disease group only was obtained through comprehensive Mayo medical records and a study-specific questionnaire. The presence of any hyaline fibrous involution lobule(s) and the within-sample proportion of hyaline fibrous involution lobules relative to the total number of lobules were compared between groups.
Statistical analysis
Number of hyaline fibrous involution lobules was analyzed as a count variable using a negative binomial regression model with the logarithm of the total number of lobules as offset and a covariate for group (BRCA vs. benign breast disease). Multivariable models additionally adjusting for degree of age-related lobular involution were also fit, as were models assessing impact of factors such as type of mutation (BRCA1 vs. BRCA2) within the BRCA subset. For the presence of any hyaline fibrous involution lobules within the sample, logistic regression adjusted for total lobules was used to compare groups. Negative binomial regression models were fit using the glm.nb function from the MASS library [9] for R (www.r-project.org). Among the benign breast disease subjects, the association of hyaline fibrous involution with future breast cancer events was assessed with Fine and Gray competing risks regression using the cmprsk package [10]. p-values < 0.05 were considered statistically significant.
Histochemical and immunohistochemical staining
We selected 19 samples with hyaline fibrous involution (14 BRCA and five benign breast disease), for immunostains to investigate the composition of the hyalinized basal lamina-like material in the hyaline fibrous involution lobules. A representative block was chosen from each case to stain with Masson-trichrome, elastin, and collagen type IV. Immunohistochemical stains were performed on four micron sections cut from formalin-fixed, paraffin-embedded blocks, which were placed on “charged” slides; slides were dried and melted in a 62 °C oven for twenty minutes. Slides were placed on a Ventana BenchMark XT (Ventana Medical Systems Inc.) for staining. Immunohistochemistry was performed with a mouse monoclonal antibody to collagen IV (clone CIV22; 1/50 dilution) (Dako, Carpinteria CA). The staining protocol includes online deparaffinization, HIER (Heat Induced Epitope Retrieval) with Ventana Cell Conditioning 1 Mild, primary antibody incubation for 32 minutes at 37 °C. Antigen–antibody reactions are visualized using the Ventana UltraviewTM Universal DAB Detection Kit. Counterstaining is performed online using Ventana Hematoxylin II for 8 min, followed by bluing reagent for 4 min. Slides are removed from the stainer, dehydrated, and coverslipped for microscopic examination.
Results
Patient sample
Among 93 women with BRCA mutations, 58 (62%) were BRCA1 mutations and 35 (38%) were BRCA2 mutations. Mastectomies were bilateral prophylactic in 33 (36%) and contralateral prophylactic in 60 (65%) patients with concurrent or prior history of breast cancer on the opposite side. Median age was 45 years with a range from 25 years to 72 years for both BRCA and benign breast disease groups. Although the two groups were age-matched, they did differ significantly on degree of age-related lobular involution (p = 0.001) with complete involution in 29% of BRCA subjects vs. 13% of the benign breast disease subjects, no involution in 12% of BRCA subjects vs. 31% of the benign breast disease subjects, and partial involution for similar percentages (59% of BRCA, 56% of benign breast disease).
Comparing hyaline fibrous involution measures between BRCA and benign breast disease groups
The median total number of lobules per sample was 106 (range, 7–480) in the BRCA group prophylactic mastectomy tissues and median 62 (range, 2–321) in the benign breast disease group benign biopsy tissues, p < 0.001. Presence of any hyaline fibrous involution lobule(s) was significantly more frequent in the BRCA samples compared to the benign breast disease samples, 47% (44/93) vs. 15% (14/93) (Fig. 3). After adjusting for the total number of lobules in each sample, the odds ratio for the presence of any hyaline fibrous involution lobule(s) in the sample was 4.5 (95% CI: 2.2–9.2, p < 0.001) for BRCA vs. benign breast disease samples. Considering the number of hyaline fibrous involution lobules in the BRCA group, the within-sample proportion of hyaline fibrous involution-positive lobules relative to total lobules was mean 0.04 (SD 0.08), while hyaline fibrous involution-diffuse lobules and hyaline fibrous involution-focal lobules had mean proportions of 0.03 (SD 0.07) and 0.01 (SD 0.02), respectively. In the benign breast disease group, the mean proportion of positive for hyaline fibrous involution was 0.0076 (SD 0.03), with mean proportion hyaline fibrous involution-diffuse and hyaline fibrous involution-focal lobules of 0.0053 (SD 0.02) and 0.0023 (SD 0.01), respectively. The count of hyaline fibrous involution lobules relative to total lobules was statistically different between the BRCA and benign breast disease subjects after adjusting for total number of lobules in the sample (hyaline fibrous involution-positive lobules, p < 0.001; hyaline fibrous involution-diffuse lobules, p < 0.001; and hyaline fibrous involution-focal lobules, p = 0.003). These differences each remained statistically significant after adjustment for degree of age-related lobular involution. In the subset who did have any hyaline fibrous involution (44/93 BRCA and 14/93 benign breast disease samples), the percent of hyaline fibrous involution lobules relative to total lobules was low in both groups: median 0.03 (range, 0.004–0.45) for BRCA specimens and median 0.03 (range, 0.01–0.18) for benign breast disease, which was not significantly different (p = 0.14; Table 1).
Frequency of hyaline fibrous involution in the BRCA and benign breast disease groups. Forty-four of 93 cases (47%) in BRCA group showed hyaline fibrous involution as compared to 14 of 93 cases (15%) in the benign breast disease group, which was a statistically significant difference. After adjusting for the total number of lobules in each sample, the odds ratio was 4.5 (95% CI: 2.2–9.2, p < 0.001) for presence of any hyaline fibrous involution for the BRCA vs. benign breast disease samples
Comparing hyaline fibrous involution measures between BRCA or benign breast disease and reduction mammoplasty groups
Hyaline fibrous involution was assessed on a separate normal comparison group comprised of 86 reduction mammoplasty sample from patients with an age distribution similar (median 44 years, range 27–77 years) to the BRCA and benign breast disease groups. These reduction mammoplasty specimens were all confirmed to have either no histologic abnormality (16%) or only non-proliferative benign breast changes (84%). Only 1/86 (1%) reduction mammoplasty specimens had any hyaline fibrous involution lobules represent; this incidence was significantly lower than observed for BRCA (p < 0.001) and benign breast disease samples (p = 0.02), which demonstrated the presence of at least one hyaline fibrous involution lobule in 47% and 15% of samples, respectively.
Association of hyaline fibrous involution with other variables
Within the BRCA subjects, there was no significant difference in number of hyaline fibrous involution based on BRCA1 vs. BRCA2 mutations (p = 0.54) or whether the patient was undergoing bilateral prophylactic mastectomy vs. contralateral prophylactic mastectomy (p = 0.24). However, the presence of any hyaline fibrous involution lobule(s) within the sample was significantly more frequent in the perimenopausal age group (45–55 years: 63%) compared to other age groups (<45 years, 44%; >55 years, 15%), p = 0.05 and p = 0.02, respectively (Table 2). Also within the BRCA group, those with either partial (p = 0.008) or complete (p = 0.01) age-related lobular involution showed significantly higher proportion of hyaline fibrous involution lobules compared to subjects where involution was classified as none. In the benign breast disease group, there was no apparent association between age and presence of any hyaline fibrous involution lobules or the number of hyaline fibrous involution lobules. However, the presence of any hyaline fibrous involution lobule and the number of hyaline fibrous involution-positive lobules per total lobules did differ by age-related lobular involution in benign breast disease subjects and was significantly higher for those with partial compared to those with complete and none (p = 0.03 and p = 0.002, respectively).
Hyaline fibrous involution and breast cancer risk
During a median of 16 years follow-up, 12 of 93 cases in the benign breast disease group developed breast cancer. On univariate analysis, presence of any hyaline fibrous involution-positive lobule(s) in women with benign breast disease showed a HR of 2.06 (95% CI: 0.49–8.64, p = 0.32), but this effect size was attenuated when adjusted for age (HR 1.49, 95% CI: 0.46–4.88, p = 0.51).
Special stains
All 14 cases stained showed that the hyalinized basal lamina-like material in hyaline fibrous involution stained blue in Masson-trichrome stain (Fig. 2f), and all were negative for elastin and collagen type IV. No differences were observed between BRCA and hyaline fibrous involution samples. These findings indicate that the hyaline material in hyaline fibrous involution lobules is composed of type I collagen.
Discussion
Our data demonstrate that hyaline fibrous involution comprise a variable but relatively small proportion of total terminal duct lobular units in any representative breast sample. However, it is approximately threefold more likely to be present in women with BRCA mutation compared to age-matched women with benign breast disease, and only rarely (1%) seen in age-matched women with no histologic abnormalities or non-proliferative changes of the breast. Hyaline fibrous involution is seen in a small terminal duct lobular unit with atrophic epithelium, but it is histologically different from age-related lobular involution by virtue of its characteristic periacinar symmetric, regular deposition of basal lamina-like material which was found to be type I collagen by Masson-trichrome stain.
Although BRCA2-mutation associated breast cancers are not significantly different than sporadic cancers with respect to pathologic parameters, BRCA1-mutated breast cancers are typically triple-negative (ER-negative, PR-negative, HER2-negative) with high grade “basal-like” phenotype, pushing margins, and lymphocytic infiltrates [4]. Efforts to identify histopathologic features in non-neoplastic breast parenchyma that characterize or predict BRCA mutation status have yielded controversial results. Some studies indicate that high-risk (atypical) breast lesions are more common in BRCA mutation carriers than in patients with sporadic breast cancer [11,12,13,14]. Others have shown that women with BRCA mutation had a lower prevalence of proliferative fibrocystic changes [15]. At the present time, there is no generally accepted finding in benign breast tissue that reliably reflects BRCA mutation (i.e., in the absence of cancer).
Bayraktar et al. [16] detailed histological features of peritumoral non-neoplastic parenchyma in women with and without BRCA mutation. They assessed six histologic features such as benign proliferative breast disease with and without atypia, stromal fibrosis, lobular type, lobular contour, lobular sclerosis, and lobulitis. These authors concluded that there is no significant association between mutation status and benign histologic features in peritumoral breast parenchyma. Lobular contour, in their report, was defined as “regular” if the distinct lobular architecture was preserved with smooth borders of non-neoplastic lobules. They indicated that the prevalence of regular lobular contour was slightly higher in the BRCA-mutated group than in the BRCA-wild group (61% vs. 52%) although it was not statistically significant. Based on their histologic description and microphotograph published, we believe at least a part of the cases with regular lobular contour might represent hyaline fibrous involution.
Russo et al. [17] have described three “developmental patterns” of breast lobules. One of them (Lob 1 in their terminology) is characterized by clusters of 6–11 “ductules” per lobule, with intralobular fibrosis and hyalinization. These authors showed that Lob 1 pattern is more common in postpubertal nulliparous women and patients with BRCA mutation (regardless of parity). They suggested that Lob 1 pattern represents a “failure of differentiation” and is associated with increased risk of breast cancer. Based on the photomicrograph published in that paper, we believe that their Lob 1 pattern may represent what we are calling hyaline fibrous involution, a conclusion substantiated by a common association with BRCA mutational carrier status. Whether the presence of characteristic periacinar hyaline material reflects abnormal development vs. altered involution is unknown. However, the perimenopausal age association we observed in this study corresponds to the conclusion of physiological involution, suggesting an acquired rather than developmental etiology.
A number of recent publications implicate the importance of tumor microenvironment in tumor development and progression [18], some suggesting that DNA instability of stromal cells plays a role in carcinogenesis. Interestingly, Salem et al. [19] demonstrated that BRCA1-knock out fibroblasts induced a 2.2-fold increase in breast tumor growth. They addressed upregulation of HIF-1α, autophagy, and ketone body formation as a possible mechanism for the phenomenon. Given these findings, there might be a possible link between BRCA mutation and increased collagen deposition/stromal fibrosis shown in the present study. Furthermore, mammographically dense breast tissue has been recognized as one of the greatest risk factors for developing breast carcinoma, where areas of mammographically dense breast tissue show not only increased epithelial and stromal cellularity, but also increased fibrillary collagen deposition.
Provenzano et al. [20] demonstrated that increased stromal collagen in mouse mammary tissue significantly increases tumor formation approximately threefold using a bi-transgenic tumor model with increased tumor stromal collagen in mouse mammary tissue, which offers some possible clues to explain why hyaline fibrous involution may be associated with increase breast cancer risk. They suggested two possible mechanisms for this phenomenon. First, increased breast density is related to a stiffer extracellular matrix conferring high local mechanical loads and higher resistance to cellular contractility for breast epithelial cells. Such changes have been shown to alter focal adhesion and Rho GTPase signaling, resulting in increased proliferation and phenotypic transformation of epithelial cells. Second, stromal fibroblasts may regulate epithelial cells in part through secretion of specific soluble growth factors and chemokines such as TGF-beta, EFGR, HER2, and insulin-like growth factors.
To our knowledge, this is the first paper to quantify presence of hyaline fibrous involution. Two papers mentioned above [16, 17] included photomicrographs showing strikingly similar histologic features to hyaline fibrous involution, but they did not describe histologic findings in detail to characterize this as a new entity. Also, it could be argued that hyaline fibrous involution resembles so called hyaline sclerosing lobular hyperplasia (fibroadenomatoid mastopathy). Hyaline sclerosing lobular hyperplasia is found in breast tissue surrounding about 50% of fibroadenomas and most phyllodes tumors. However, fibroadenomatoid mastopathy and hyaline fibrous involution are distinct; fibroadenomatoid mastopathy is characterized by enlarged lobules composed of an increased number of acini, whereas hyaline fibrous involution lobules are smaller and contain fewer acini than normal lobules.
Based on our findings, we further theorize that hyaline fibrous involution might reflect a form of pathologic involution, possibly caused by or associated with cellular injury. This would account for its more frequent but not exclusive presence in the setting of BRCA mutation. Therefore, we hypothesize that hyaline fibrous involution may represent a previously uncharacterized, preneoplastic benign terminal duct lobular unit phenotype associated with an increased risk of breast cancer regardless of BRCA mutation status. More studies involving larger cohorts and ancillary studies will be required.
References
Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–7.
Radisky DC, Hartmann LC. Mammary involution and breast cancer risk: transgenic models and clinical studies. J Mammary Gland Biol Neoplasia. 2009;14:181–91.
Radisky DC, Visscher DW, Frank RD, Vierkant RA, Winham S, Stallings-Mann M, et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Res Treat. 2016;155:423–30.
Golgar D, Devilee P. Genetic susceptibility: inherited syndromes. BRCA1 and BRCA2 syndromes. In: Lakhani SR, Schnitt SJ, Tan PH, van de Vijver MJ, editors. WHO classification of tumours of the breast. 4th edn. Lyon, France: International Agency for Research on Cancer; 2012. p. 176–82.
Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291–9.
Venkitaraman AR. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu Rev Pathol. 2009;4:461–87.
Hartmann LC, Lindor NM. The role of risk-reducing surgery in hereditary breast and ovarian cancer. N Engl J Med. 2016;374:454–68.
Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–37.
Venables WN, Ripley BD. Modern Applied Statistics with S. 4th edn. New York: Springer; 2002. p. 206–7.
Gray B. cmprsk: Subdistribution analysis of competing risks. R package version 2.2-6. 2013. http://CRAN.R-project.org/package=cmprsk.
Hoogerbrugge N, Bult P, de Widt-Levert LM, Beex LV, Kiemeney LA, Ligtenberg MJ, et al. High prevalence of premalignant lesions in prophylactically removed breasts from women at hereditary risk for breast cancer. J Clin Oncol. 2003;21:41–5.
Leunen K, Drijkoningen M, Neven P, Christiaens MR, Van Ongeval C, Legius E, et al. Prophylactic mastectomy in familial breast carcinoma. What do the pathologic findings learn us? Breast Cancer Res Treat. 2008;107:79–86.
Kauff ND, Brogi E, Scheuer L, Pathak DR, Borgen PI, Hudis CA, et al. Epithelial lesions in prophylactic mastectomy specimens from women with BRCA mutations. Cancer. 2003;97:1601–8.
Arun B, Vogel KJ, Lopez A, Hernandez M, Atchley D, Broglio KR, et al. High prevalence of preinvasive lesions adjacent to BRCA1/2-associated breast cancers. Cancer Prev Res (Phila). 2009;2:122–7.
Adem C, Reynolds C, Soderberg CL, Slezak JM, McDonnell SK, Sebo TJ, et al. Pathologic characteristics of breast parenchyma in patients with hereditary breast carcinoma, including BRCA1 and BRCA2 mutation carriers. Cancer. 2003;97:1–11.
Bayraktar S, Qiu H, Liu D, Shen Y, Gutierrez-Barrera AM, Arun BK, et al. Histopathological features of non-neoplastic breast parenchyma do not predict BRCA mutation status of patients with invasive breast cancer. Biomark Cancer. 2015;7:39–9.
Russo J, Lynch H, Russo IH. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2001;7:278–91.
McCready J, Arendt LM, Rudnick JA, Kuperwasser C. The contribution of dynamic stromal remodeling during mammary development to breast carcinogenesis. Breast Cancer Res. 2010;12:205.
Salem AF, Howell A, Sartini M, Sotgia F, Lisanti MP. Downregulation of stromal BRCA1 drives breast cancer tumor growth via upregulation of HIF-1alpha, autophagy and ketone body production. Cell Cycle. 2012;11:4167–73.
Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work has been, in part, presented at the Annual United States and Canadian Academy of Pathology meeting in March, 2016 in Seattle, WA, USA.
Rights and permissions
About this article
Cite this article
Lee, H.E., Arshad, M., Brahmbhatt, R.D. et al. Hyaline fibrous involution of breast lobules: a histologic finding associated with germline BRCA mutation. Mod Pathol 32, 1263–1270 (2019). https://doi.org/10.1038/s41379-019-0217-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0217-9