Abstract
To determine whether tumor microenvironments affect the clinical response to anti-PD-1 therapy in non-small cell lung cancer, we investigated the expression level of PD-L1 and tumor infiltrating lymphocytes and elucidate their predictive role. Thirty-eight pretreatment and two post-treatment specimens from 36 advanced, treatment-refractory non-small cell lung cancer patients who underwent PD-1 blockade therapy were analyzed. PD-L1 expression by tumor cells and the distribution of CD3, CD8, CD4, FOXP3 and PD-1 positive tumor infiltrating lymphocytes were immunohistochemically assessed and counted using digital image analyzer. CD3+ and CD8+ T cells were distributed more in PD-L1 positive group compared to PD-L1 negative group. Conversely, EGFR mutant group showed fewer CD3+ T cells than EGFR-naïve group. The patients in the clinical benefit group with PD-1 blockade showed a higher number of CD3+, CD8+ T cells and a higher CD8+/CD3+ T cell ratio (p = 0.003, p = 0.001, and p = 0.042) and a lower FOXP3+/CD8+ T cell ratio compared to non-responders (p = 0.001). In multivariate logistic regression analysis, increased CD3+ T cell infiltration and low FOXP3+/CD8+ T cell ratio were found to be independent predictors of clinical benefit with PD-1 blockade (p = 0.014 and p = 0.03, respectively). Using receiver operating characteristic curves, levels of CD3+ T cells and FOXP3+/CD8+ T cell ratio that provide the best distinguishing point between responder versus non-responder to PD-1 blockade were 617.5/mm2 and 25%, respectively (p = 0.007 and p = 0.003). Considering that 1 mm2 is about 5 high power fields (HPF), a good response to the PD-1 blockade can be expected when the number of CD3 T cells is observed to be 120 per HPF and when CD8+ T cells and FOXP3+ T cells are present at a ratio greater than 4:1. Tumor infiltrating lymphocytes might become a promising biomarker as an independent predictive factor of response to PD-1 blockade that may also guide therapeutic decisions.
Similar content being viewed by others
Introduction
Although programmed cell death ligand-1 (PD-L1) expression in tumor cells potentially enriches the responders to immune checkpoint inhibitors in non-small cell lung cancer, it is not an absolute predictor of therapeutic response [1, 2]. Several large harmonization studies were performed to overcome the assay variability, and as a result, methodological issues no longer seem to be an excuse for the low predictive value of PD-L1 [3,4,5]. Rather, the variability in PD-L1 expression in non-small cell lung cancer is due to intrinsic biologic and genetic factors, such as smoking and EGFR and KRAS mutations, and it is implicated in tumor immunogenic properties [6, 7].
In addition to PD-L1 expression, other intratumoral factors have been proposed as predictive biomarkers for anti-PD-1 therapy outcomes. One of these factors is tumor-infiltrating lymphocyte, which contribute critical information that can aid interpretation of tumor-immune system reactions [8,9,10]. The balance between PD-L1–mediated tumor immune escape and an efficient immune reaction against cancer induced by tumor infiltrating lymphocytes may provide highly relevant therapeutic information. Furthermore, tumor infiltrating lymphocytes are potentially easier to detect and quantify in pathological specimens.
Many studies have used immunohistochemistry to define T cell subsets, including CD3+, CD8+, CD4+, and FOXP3+ subsets, and investigated their prognostic implications [11,12,13,14]. Only few studies have addressed the predictive effect of tumor infiltrating lymphocytes in non-small cell lung cancer patients [9, 10]. In addition, these studies examined small numbers of PD-1 blockade treated patients, heterogenous tumor types, and could not provide a cutoff value for tumor infiltrating lymphocytes that could predict the responder group [9, 10].
The present work was conducted to determine whether tumor infiltrating lymphocytes influence the response to anti-PD-1 therapy in non-small cell lung cancer patients. In addition, we tried to calculate a cutoff value for tumor infiltrating lymphocytes that could predict the responder group and allow tumor infiltrating lymphocytes to be used as a predictive marker in the clinic.
Materials and methods
Study population
We selected advanced non-small cell lung cancer patients who were administered PD-1 blockade therapy and evaluated for their drug response from May 2016 to February 2018 at our institution and had available pretreatment tumor specimens. Finally, this study included a total of 36 patients. Of these, 32 patients had 1 pretreatment sample, 2 patients had 2 pretreatment samples from different time periods, and the other 2 patients had 2 samples acquired before and after receiving the PD-1 blockade treatment. Therefore, all 36 patients had at least one pretreatment sample, and the total number of pretreatment samples used in this study was 38. Two post-PD-1 blockade-treated samples were also analyzed separately. This study was approved by the institutional review board of Seoul National University Bundang Hospital (B-1803-456-304), which approved the patient consent forms or in some cases the waiver of consent.
Smoking history, histological diagnosis, pathological staging, and genetic alteration status were obtained from medical records. Patients treated with PD-1 blockade were placed in the responder group (complete response, partial response, or stable disease persisting for at least 6 months) according to RECIST criteria version 1.1 [15]. Specimen acquisition dates ranged from May 2012 to December 2017, with a mean time from acquisition to immunohistochemical staining of 15.7 months and a median of 11.5 months (range 0–67 months).
Automatic immunohistochemical staining and scoring of tumor infiltrating lymphocytes
Two board-certified pathologists (H.K and J.H.C) reviewed formalin-fixed paraffin-embedded tissue sections stained with hematoxylin and eosin (H&E) to confirm the pathologic diagnosis and selected a representative paraffin block from each specimen for immunohistochemical analysis of surgically resected samples. In the case of small biopsy specimens, we included only core needle or bronchoscopic biopsy specimens with a size of at least 10 mm, and to select representative samples from the tumor microenvironment, we excluded biopsy samples that encompassed only the peritumoral stroma or <50% of tumor tissue. Endobronchial ultrasound guided biopsy or cytology specimens were excluded.
Immunohistochemical staining was carried out with a VENTANA XT autostainer (Ventana, Tucson, AZ) using 4-μm-thick sectioned specimens. The five antibodies and conditions used in this study—anti-CD3, CD4, CD8, FOXP3, and PD-1—are listed in the Supplementary Table 1. All stained slides were scanned using a high-resolution digital slide scanner up to 400x magnification (3DHISTECH Pannoramic 250; 3DHISTECH Ltd., Budapest, Hungary) and counted automatically by a computerized image analysis system (QuantCenter 2.0; 3DHISTECH Ltd., Budapest, Hungary). The densities of cells expressing CD3, CD4, CD8, FOXP3, and PD-1 were evaluated using NuclearQuant software that counted the positive cells throughout the entire tumor area but not in tissue outside the tumor border (Supplementary Figure 1). The mean number of cells positive for each marker is expressed as density per mm2. In addition, CD8+/CD3+, CD8+/CD4+, FOXP3+/CD8+, and PD-1+/CD8+ ratios were calculated for each specimen using the number of total fields, and the averages were compared.
PD-L1 protein expression
PD-L1 immunohistochemistry was performed with 22C3 and/or SP263 assays before immune checkpoint inhibitor treatment for all patients as previously described [16]. 10 of 40 (25%) specimens were tested with both 22C3 and SP263 assays, 12 (30%) with only the 22C3 assay, and 18 (45%) with only the SP263 assay. PD-L1 expression was evaluated in tumor cells and is reported as the tumor proportion score [17, 18]. Regardless of the assay, PD-L1 positive was defined as a tumor proportion score of more than 1%, and cases with a tumor proportion score of more than 50% in the 22C3 assay or more than 25% in the SP263 assay were further classified as strongly PD-L1 positive [18, 19].
Histologic assessment of tumor infiltrating inflammatory cells and stromal fibrosis
We analyzed the tumor infiltrating inflammatory cells for only 33 primary lung lesions, considering that the microenvironment of the metastatic site would affect the tumor infiltrating inflammatory cell analysis. We designated tumor infiltrating inflammatory cells as the density of the generalized inflammatory cell infiltrates (not specific T cells) that were evaluated on routine H&E staining. The percentage of tumor infiltrating inflammatory cells is a semiquantitative parameter for this assessment; for example, 50% tumor infiltrating inflammatory cells means that 50% of the stromal area shows inflammatory cell infiltrates. To assess the percentage values, the dissociated growth pattern of inflammatory cells were taken into account. Tumor infiltrating inflammatory cells in each slide were evaluated within tumor area excluding tissue outside the tumor border and intratumoral necrotic area. In addition, degree of stromal fibrosis was assessed semiquantitatively. The results are reported as the proportion of fibrosis in the stroma and were calculated as a percentage of the whole tumor field, excluding necrosis. Representative images are shown in Supplementary Table 2.
Statistical analysis
Student’s t-test, Chi-square test, and Wilcoxon rank-sum test were used to compare variables between subgroups of patients. The probability of clinical benefit with PD-1 blockade based on clinicopathologic variables was determined by univariate and multivariate logistic regression analyses. The cutoff values providing the best separation between the responders and non-responders to PD-1 blockade were determined using receiver operating characteristic curves. For survival analyses, Kaplan–Meier curves were used to estimate time to event outcome parameters, and different groups were compared using a log-rank test. All tests were two-sided, and p-values <0.05 were considered statistically significant. The data were analyzed using SPSS version 21 software (SPSS Inc., Chicago, IL).
Results
Demographics
Detailed patient and tumor characteristics are reported in Table 1. Although most of the samples were lung specimens, 5 samples were obtained from metastatic lesions (4 from lymph node, and 1 from mesentery). Regarding PD-1 blockade therapy, more than half of the patients received nivolumab, 25% received pembrolizumab, and 3 patients received sequential administration of two or more drugs, including nivolumab, pembrolizumab, and atezolizumab. Most patients who received PD-1 blockade therapy as a second-line treatment were refractory to conventional chemotherapy, radiotherapy and/or targeted therapy, and only one received first line therapy. The majority of cases were positive for PD-L1 immunoreactivity (82%), and 61% showed strong PD-L1 positivity in 22C3 or SP263 assays.
According to RECIST guidelines version 1.1 [15], thirteen patients (36%) were sorted into the clinical benefit group, while the remaining 23 patients were considered non-responders (Table 1). Among the 22 ADC patients and 1 ASC patient, 6 EGFR-mutated patients and 1 ALK-rearranged patient received tyrosine kinase inhibitor therapy before PD-1 blockade.
Tumor infiltrating lymphocytes and clinicopathologic factors
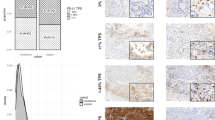
The absolute number of tumor infiltrating lymphocytes differed according to PD-L1 expression, EGFR mutation status, and percentage of tumor infiltrating inflammatory cells based on H&E staining (Fig. 1a) but was not associated with other factors, such as age, sex, smoking history, and histologic subtype. High numbers of infiltrating CD3+ and CD8+ T cells were identified in PD-L1-positive specimens and the tumor infiltrating inflammatory cells ≥50% group compared with those in the PD-L1-negative specimens and the tumor infiltrating inflammatory cells <50% group, respectively. Inversely, the EGFR mutant group showed a low number of CD3+ T cells compared with the number in the EGFR-naïve group (p = 0.006), and the ratio of tumor infiltrating lymphocytes was not different between the groups.
a The absolute numbers of tumor infiltrating lymphocytes according to the expression of PD-L1, EGFR mutation status, and percentage of tumor infiltrating inflammatory cells evaluated on H&E section. b Association between tumor infiltrating lymphocytes and response to PD-1 blockade. Patients in the PD-1 blockade clinical benefit group showed a higher number of CD3+ and CD8+ T cells, higher CD8+/CD3+ T cell ratios, and lower FOXP3+/CD8+ T cell ratios than non-responders. c Representative tumor infiltrating lymphocyte and PD-L1 immunohistochemical images from responders (upper, x10 magnification) and non-responders (lower, x10 magnification) with PD-1 blockade treatment. The numbers of CD3+ and CD8+ T cells were increased in the tumor area of responders compared with non-responders. The number of FOXP3+ T cells was similar in the two groups. Notably, the expression of PD-L1 on tumor cells was higher in non-responders than responders
There was not a significant relationship between sample size (resection vs. biopsy) and tumor infiltrating lymphocytes expression (Supplementary Table 3A). In addition, obtaining the specimen from the primary site vs. a metastatic lesion site did not affect the expression of tumor infiltrating lymphocytes (Supplementary Table 3A). The period from specimen acquisition to PD-1 blockade administration varied from 48 months to 3 days; therefore, we investigated whether the change in tissue antigenicity affected the interpretation of tumor infiltrating lymphocytes. Therefore, we investigated TIL expression in groups of samples acquired at intervals of 3, 6, and 12 months prior to PD-1 blockade treatment; we found that time interval between sample acquisition and staining did not influence TIL expression (Supplementary Table 2).
Associations between tumor infiltrating lymphocytes, clinicopathologic parameters, and response to PD-1 blockade
Patients in the PD-1 blockade clinical benefit group showed a higher number of CD3+ and CD8+ cells, higher CD8+/CD3+ T cell ratios (p = 0.003, p = 0.001, and p = 0.042), and lower FOXP3+/CD8+ T cell ratios than non-responders (p = 0.001) (Fig. 1b, c).
We analyzed the effects of tumor infiltrating lymphocytes and clinicopathologic factors on the response to PD-1 blockade. For the 2 patients with 2 pretreatment samples, we performed analysis using the sample that showed higher tumor infiltrating lymphocytes than the other. In univariate analysis, high CD3+ and CD8+ T cell infiltration was found to be a predictor of clinical benefit from anti-PD-1 therapy (p = 0.017 and p = 0.037, respectively) (Table 2). Although the number of FOXP3+ cells was not associated with drug response, a low FOXP3+/CD8+ T cell ratio was associated with clinical benefit from anti-PD-1 therapy (p = 0.027). The PD-1+/CD8+ ratio showed a tendency to be a negative predictor, but this trend was not statistically significant (p = 0.087). The density of CD4+ and PD-1+ cells and PD-L1 expression on tumor cells were not associated with anti-PD-1 therapeutic response. The percentage of tumor infiltrating inflammatory cells and stromal fibrosis examined in H&E slides were not associated with anti-PD-1 therapeutic response. Clinicopathologic parameters, including age, sex, smoking history, histologic subtype, genetic status, type of anti-PD-1 drug, and PD-L1 expression, were not correlated with response to PD-1 blockade (Table 2). In multivariate analysis, number of CD3+ T cells and the FOXP3+/CD8+ T cell ratio were independent predictors of PD-1 blockade treatment response (Table 2).
Changes in tumor infiltrating lymphocyte and PD-L1 expression in four patients with paired samples
We assessed the expression of tumor infiltrating lymphocytes and PD-L1 in eight samples from four patients with paired samples acquired at different time points and compared the PD-1 blockade response results. Clinical information for the paired samples and tumor infiltrating lymphocytes and PD-L1 expression are described in Supplementary Table 3.
Tumor infiltrating lymphocytes infiltration was increased or decreased randomly regardless of subpopulation, and the differences between the previous and recent samples were not consistent. The results in the two patients with samples before and after PD-1 blockade treatment should be noted (patients nos. 3 and 4 in Supplementary Table 3). In patient no. 3, tumor infiltrating lymphocytes infiltration in both the pre- and post-treatment samples was significantly lower than that in patients 1 and 2, who were both responders. In particular, the FOXP3+/CD8+ T cell ratio was greatly increased with the decrease in CD8+ T cells after treatment. The expression of PD-L1 also changed from negative to positive. In patient no. 4, the expression of tumor infiltrating lymphocytes was increased after treatment, but the expression of PD-L1 was decreased. These two patients were non-responders to PD-1 blockade therapy, and their pre-immune checkpoint inhibitor treatment sample showed less CD3+ and CD8+ T cell infiltration and a higher FOXP3+/CD8+ T cell ratio than that of responders.
Selection of tumor infiltrating lymphocyte cut-off point
The receiver operating characteristic curves for the number of CD3+ T cells and the FOXP3+/CD8+ T cell ratio clearly illustrate the point on the curve closest to (0.0, 1.0) that maximizes both PD-1 blockade response sensitivity and specificity (Fig. 2a, b). The level of CD3+ T cells and the FOXP3+/CD8+ T cell ratio that provide the best discrimination point between responders and non-responders were 617.5/mm2 and 25%, respectively (p = 0.007 and p = 0.003). The sensitivity, specificity and area under the curve (AUC) was 84.6%, 65.2%, and 0.774 for CD3+ T cells and 82.6%, 65.4%, and 0.799 for the FOXP3+/CD8+ T cell ratio, respectively. Considering that 1 mm2 is approximately 5 high power fields (HPFs), a good response to the PD-1 blockade can be expected when the number of CD3+ T cells is observed to be 120 per 1 HPF and when CD8+ T cells and FOXP3+ T cells are present at a ratio greater than 4:1.
a, b Receiver operating characteristic curves for classifying responder versus non-responder with PD-1 blockade in non-small cell lung cancer in whole cohort. a The number of CD3+ T cells attained average AUC of 0.774. b FOXP3+/CD8+ T cell ratio attained average AUC of 0.799. c, d Receiver operating characteristic curves for classifying responder versus non-responder with PD-1 blockade in subgroup without driver mutation (EGFR and ALK). c The number of CD3+ T cells attained average AUC of 0.758. b FOXP3+/CD8+ T cell ratio attained average AUC of 0.802
We also analyzed the cut-off values of CD3+ T cells and the FOXP3+/CD8+ T cell ratio after eliminating 7 EGFR-mutated and 1 ALK-rearranged patients because these driver mutations are already considered negative predictive factors for immune checkpoint inhibitor response. [20, 21] The results were similar with those of the whole cohort (628.5/mm2 for CD3+ T cells with p = 0.022 and 24% for the FOXP3+/CD8+ T cell ratio with p = 0.007) (Fig. 2c, d).
Survival analysis
Patients who showed a clinical benefit from PD-1 blockade tended to have better overall survival than the disease progression group, although this difference was not statistically significant (p = 0.226; Supplementary Table 3). However, in general, other clinicopathologic variables, including tumor infiltrating lymphocytes and PD-L1 expression, were not prognostic indicators.
Discussion
In this study, we found that tumor infiltrating lymphocytes were an independent predictive factor of PD-1 blockade treatment response, and we presented a cutoff value for tumor infiltrating lymphocytes to discriminate between responder and non-responder groups. In addition, we demonstrated that tumor infiltrating lymphocytes status evaluated in biopsy samples and archival tissues is a feasible approach for predicting therapeutic response.
Examination of the tumor microenvironment in patients treated with immune checkpoint inhibitors has revealed an association between the tumor infiltrating lymphocytes count both before and after treatment and therapy response [8, 22]. Recently, in addition to evaluation of tumor infiltrating lymphocytes based on H&E or immunohistochemical staining, studies were conducted to evaluate tumor infiltrating lymphocytes using a developed a multiplex technique. [23] However, this method cannot be routinely used for evaluation of tumor infiltrating lymphocytes in most pathology laboratories because of the high cost of equipment and the complicated technology, and there is still a need technical verification. Therefore, we tried to evaluate the number of tumor infiltrating lymphocytes as objectively as possible using a method that can be applied in routine practice and used this method as an index to predict PD-1 blockade response. As a result, CD3+ T cells greater than 120 per HPF, and CD8+/FOXP3+ T cell ratios greater than 4:1 were found to be associated with clinical benefit in PD-1 blockade treated patients.
The binding of PD-1 to PD-L1 causes T cell to become anergy and contributes to the immune escape of tumor cells [24,25,26]. CD8+ cytotoxic tumor infiltrating lymphocytes can kill tumor cell directly and general T-cells expressing pan T cell marker, CD3, interplay with different subsets of immune cells via cytokine such as INFγ [24, 27]. Our study results are in line with the results of Schalper et al. [23], who noted that the presence of elevated CD3+ and CD8+ T cells is consistently associated with survival and reported that CD8 was the only independent prognostic factor in non-small cell lung cancer. Taken together, these tumor specific CD3+ and CD8+ subsets of T cells were involved in adaptive immunity and might be important not only as a prognostic marker but also a predictive biomarker for PD-1 blockade in non-small cell lung cancer.
In addition, we should note the role of FOXP3+ tumor infiltrating lymphocytes in immunotherapy; the CD8+/FOXP3+ T cell ratio was a more important factor in predicting immune checkpoint inhibitor response than the absolute number of CD8+ T cells. Accumulating studies have demonstrated a large number of tumor-infiltrating FOXP3+ Tregs in various tumors. In breast [28], gastric [29], and ovarian cancer [30]; in the majority of solid tumors in the cervix, kidney, and breast; and in melanoma, decreased ratios of tumor-infiltrating CD8+ T cells to FOXP3+ Tregs or increased tumor-infiltrating FOXP3+ cells were shown to be correlated with poor prognosis [28]. In contrast, FOXP3+ tumor infiltrating lymphocytes in colorectal cancer were heterogeneously present in both the suppressive and non-suppressive forms, and the effect of FOXP3+ tumor infiltrating lymphocytes on prognosis is controversial [31,32,33]. Based on our results, FOXP3+ tumor infiltrating lymphocytes in non-small cell lung cancer might have a suppressive function similar to that found in other solid tumors and may inhibit the cytotoxic activity of the accompanying CD8+ T cells and affect the immunotherapy response [34]. In vitro coexistence of Tregs suppresses nivolumab‐induced release of interferon‐γ from effector T cells [35]. In short, Tregs may interfere with the immunostimulatory effects of PD‐L1 inhibitors in non-small cell lung cancer.
In most cases (31/38, 82%), tumors were positive for PD-L1, and the PD-L1-positive tumors were more likely to contain tumor infiltrating lymphocytes than PD-L1-negative tumors. Several studies have reported that PD-L1 is more frequently expressed in such a favorable immune microenvironment [36,37,38], which supports the notion that PD-L1 expression by tumors is a mechanism of adaptive immune resistance. However, PD-L1 expression was not a predictor of PD-1 blockade response. This finding was similar to the results of the checkmate 017 and 057 trials, suggesting that the majority of patients in our cohort used nivolumab, and thus, PD-L1 expression had little effect on immune checkpoint inhibitor response [17, 39]. We think that the lack of an association between PD-L1 expression and drug response is likely not related to performance of the PD-L1 assay but is rather a function of the complex interactions between tumors and the immune system. In this context, the evaluation of tumor infiltrating lymphocytes is a more important key factor in accurately predicting immune checkpoint inhibitor response in patients with PD-L1 expression and could compensate for the limitations of PD-L1 expression.
The second major outcome of our study was demonstration of the feasibility of using biopsy specimens to evaluate tumor infiltrating lymphocytes. In examining biopsy specimens from tumors, the representativeness of a small biopsy and its ability to adequately reflect the heterogeneous tumor microenvironment has always been an issue. Obeid et al. evaluated CD8+ tumor infiltrating lymphocytes density using eight sampling strategies and examined which method resulted in measures that were most similar to those obtained with the whole tumor [40]. Although different tumor sampling strategies may yield discordant tumor infiltrating lymphocyte density results, sampling 10–20 small areas randomly, sampling the tumor center, or taking large core biopsies (10 × 1 mm) may best represent the whole tumor [40]. Based on this result, we included only biopsy tissues in which the size of the specimen was more than 10 mm and the peripheral normal tissue was nearly absent, which were most likely to be obtained from the center of the tumor. As a result, biopsy tissues showed less CD3+ and CD8+ tumor infiltrating lymphocyte density than resected tissues, but the difference was not statistically significant and consequently did not affect treatment response prediction. Considering that many lung cancer patients receiving immunotherapy have advanced stage disease, a biopsy specimen may be the only sample type that can be used to assess the patient tumor microenvironment status. Therefore, obtaining biopsy tissue of appropriate size from the center of the tumor at the time of diagnosis can be great help for predicting a patient’s immunotherapy response.
PD-1 blockade therapy seems induce a definite change in tumor infiltrating lymphocytes infiltration and PD-L1 expression in tumor cells. Tumeh et al. previously reported that a greater increase in CD8+ density from baseline to post-dosing biopsy was significantly correlated with immune checkpoint inhibitor in responding malignant melanoma patients [8]. Similar to these results, our study also showed a significant decrease in CD8+ tumor infiltrating lymphocytes in paired samples from patients who did not respond to PD-1 blockade. Another non-responder had increased CD8+ T cells after treatment, but it was difficult to make a direct comparison because the sampling sites before and after treatment were different. Nevertheless, in the pretreatment sample, the CD3+ tumor infiltrating lymphocytes number and FOXP3+/ CD8+ tumor infiltrating lymphocyte ratio were lower than the cutoff value, and the patient did not respond to the PD-1 blockade regardless of PD-L1 status. As a result, tumor infiltrating lymphocytes were confirmed to be a major predictor of PD-1 blockade response that can complement PD-L1 assessment.
There are limitations and possible bias in this study. The sample number was not large, and because of the nature of a retrospective study, it is possible that patient selection was biased according to sample availability. In addition, 4 out of 5 metastatic lesions in our cohort were in the lymph nodes, and this might have affected the proportion of tumor infiltrating lymphocytes. Although we attempted to analyze only the tumor cells and the tumor microenvironment while excluding the normal lymph node portion, there was still the possibility of bias caused by lymph node specimens. Despite these limitations, we found that tumor infiltrating lymphocyte quantitation using an immunohistochemical technique, which can be used in routine practice in patients with lung cancer treated with immune checkpoint inhibitors, is an important factor in predicting therapeutic response.
In conclusion, we evaluated tumor infiltrating lymphocytes in 38 pretreatment non-small cell lung cancer samples from immune checkpoint inhibitor-treated patients. A high number of CD3+ T cells and a low FOXP3+/CD8+ T cell ratio were identified as independent factors predicting the response to PD-1 blockade. In addition, properly sampled biopsy tissue and well-preserved archival specimens can both be useful in evaluating tumor infiltrating lymphocyte status. Based on our results, tumor infiltrating lymphocytes might become a promising biomarker that may also guide therapeutic decisions.
References
Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7.
Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–51.
Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol. 2016;29:1165–72.
Adam J, Le Stang N, Rouquette I, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol. 2018;29:953–8.
Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res. 2017;23:3585–91.
Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:860–77.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28.
Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71.
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74.
Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 expression in cytotoxic CD8(+) tumor-infiltrating lymphocytes confers an immune-privileged tissue microenvironment in non-small cell lung cancer with a prognostic and predictive value. Clin Cancer Res. 2018;24:407–19.
Geng Y, Shao Y, He W, et al. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: a meta-analysis. Cell Physiol Biochem. 2015;37:1560–71.
Zeng DQ, Yu YF, Ou QY, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget. 2016;7:13765–81.
Kim MY, Koh J, Kim S, et al. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88:24–33.
Kinoshita T, Muramatsu R, Fujita T, et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann Oncol. 2016;27:2117–23.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Kim H, Kwon HJ, Park SY, et al. PD-L1 immunohistochemical assays for assessment of therapeutic strategies involving immune checkpoint inhibitors in non-small cell lung cancer: a comparative study. Oncotarget. 2017;8:98524–32.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39.
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50.
Planchard D, Yokoi T, McCleod MJ, et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced non-small cell lung cancer: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17:232–6.e1.
Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6:e1356145.
Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22:4585–93.
Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204.
Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107:pii: dju435.
Teng MW, Ngiow SF, Ribas A, et al. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–45.
Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28:383–91.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30.
de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37.
Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–80.
Sasada T, Kimura M, Yoshida Y, et al. CD4+CD25+regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–99.
Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9.
Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92.
Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–43.
Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–84.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18.
Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–56.
Taube JM. Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade. Oncoimmunology. 2014;3:e963413.
Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer. 2016;57:91–103.
Cimino-Mathews A, Thompson E, Taube JM, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47:52–63.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Obeid JM, Wages NA, Hu Y, et al. Heterogeneity of CD8(+) tumor-infiltrating lymphocytes in non-small-cell lung cancer: impact on patient prognostic assessments and comparison of quantification by different sampling strategies. Cancer Immunol Immunother. 2017;66:33–43.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C1290 to JHC and HI18C1734 to HK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kim, H., Kwon, H.J., Han, Y.B. et al. Increased CD3+ T cells with a low FOXP3+/CD8+ T cell ratio can predict anti-PD-1 therapeutic response in non-small cell lung cancer patients. Mod Pathol 32, 367–375 (2019). https://doi.org/10.1038/s41379-018-0142-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-018-0142-3
This article is cited by
-
Generating immunogenomic data-guided virtual patients using a QSP model to predict response of advanced NSCLC to PD-L1 inhibition
npj Precision Oncology (2023)
-
Regulatory T cells induce a suppressive immune milieu and promote lymph node metastasis in intrahepatic cholangiocarcinoma
British Journal of Cancer (2022)
-
A phase I study of IMC-001, a PD-L1 blocker, in patients with metastatic or locally advanced solid tumors
Investigational New Drugs (2021)
-
Single-cell RNA sequencing reveals cellular and molecular immune profile in a Pembrolizumab-responsive PD-L1-negative lung cancer patient
Cancer Immunology, Immunotherapy (2021)
-
Expression of PD-L1, PD-L2, and IDO1 on tumor cells and density of CD8-positive tumor-infiltrating lymphocytes in early-stage lung adenocarcinoma according to histological subtype
Journal of Cancer Research and Clinical Oncology (2020)