Abstract
We describe a series of colorectal polyps characterized by mixed adenomatous and serrated features, herein referred to as superficially serrated adenomas. Twenty superficially serrated adenomas were obtained from 11 female and 9 male patients aged 62–87 years. Most lesions endoscopically appeared as small sessile polyps, but larger lesions were plaque-like (2–20 mm; median, 5 mm). Eighteen lesions (90%) were located in the sigmoid colon or rectum. They consisted primarily of straight, adenomatous glands but showed serration confined to the superficial layer. Immunohistochemistry revealed CK20 expression in the upper layer. Proliferating cells, determined by their expression of Ki-67, were localized to the middle to bottom layers. Genetic analyses identified KRAS mutations in 19 lesions and a BRAF mutation in one lesion. Furthermore, RSPO fusions and/or overexpression were observed in 18 lesions and truncating APC mutations were observed in the two remaining lesions. Consistent with the presence of WNT pathway gene alterations, all superficially serrated adenomas showed focal or diffuse nuclear β-catenin accumulation. Since concurrent KRAS mutations and RSPO fusions are reportedly common in traditional serrated adenomas, we reviewed 129 traditional serrated adenomas and found 15 lesions (12%) that were associated with superficially serrated adenoma components. Remarkably, all but one superficially serrated adenoma-associated traditional serrated adenoma exhibited concurrent KRAS mutations and RSPO fusions/overexpression. The present study suggests that superficially serrated adenoma is a morphologically and molecularly distinct type of colorectal serrated polyp that is histogenetically related to traditional serrated adenoma.
Similar content being viewed by others
Introduction
Epithelial colorectal polyps are classified into two major groups: conventional adenomas and serrated lesions [1,2,3,4]. Conventional adenomas consist of dysplastic glands and are further subclassified as tubular, tubulovillous, or villous, depending on their degree of villosity [2]. Serrated lesions are characterized by serrated crypt epithelium with or without dysplasia and include hyperplastic polyps, sessile serrated adenomas/polyps, and traditional serrated adenomas [3].
Conventional adenomas and serrated lesions are defined morphologically but also have different molecular backgrounds and are related to distinct tumorigenic pathways [1, 4, 5]. Conventional adenomas commonly have APC mutations and give rise to mismatch repair-proficient adenocarcinomas [2, 4, 6]. Serrated lesions mostly have BRAF or KRAS mutations, with dysplastic sessile serrated adenoma/polyps and traditional serrated adenomas often containing additional WNT pathway gene alterations, predominantly RNF43 mutations, and RSPO fusions [4, 5, 7,8,9,10]. Sessile serrated adenoma/polyps are recognized as precursors to BRAF-mutated adenocarcinomas with or without mismatch repair deficiency, whereas traditional serrated adenoma-derived adenocarcinomas are consistently mismatch repair-proficient [10,11,12,13,14].
Although there is still debate concerning the precise diagnostic criteria for each category, the current histological classification of colorectal polyps is generally well accepted. However, sometimes polyps with focal areas of serration are observed, which are thus difficult to classify as either a conventional adenoma or serrated lesion. A recent study analyzed a series of serrated tubulovillous adenomas and implied that they are clinicopathologically and molecularly distinct from conventional tubulovillous adenomas or traditional serrated adenomas [15]. Based on their mutation profiles and early WNT pathway activation, as indicated by nuclear β-catenin expression, serrated tubulovillous adenomas appear to be aligned more closely with the conventional pathway rather than the serrated pathway.
In the present report, we describe another previously unrecognized type of colorectal polyps showing mixed morphological features of both conventional adenomas and serrated lesions, which is herein referred to as a superficially serrated adenoma. Our study indicates that superficially serrated adenomas have a characteristic molecular background suggestive of serrated lesions and histogenetic relationship to traditional serrated adenomas.
Materials and methods
Samples
This study was approved by the Ethics Committee of the National Cancer Center, Tokyo, Japan. All tissue samples were obtained by endoscopic resection at the National Cancer Center Hospital, Tokyo, Japan, or at the National Cancer Center Hospital East, Chiba, Japan. The present study analyzed 20 colorectal superficially serrated adenomas, which consisted of adenomatous glands but showed superficial serration. Seven lesions were retrieved from the case files of S.S. The remaining 13 cases were identified through a histological review of approximately 9500 colorectal polyps, that were originally diagnosed as tubular adenoma, from the National Cancer Center Hospital, Tokyo, Japan. In addition, we histologically re-evaluated 129 traditional serrated adenomas, which had been previously characterized for their clinicopathological features and mutation profiles [8, 16], in order to determine whether any were associated with superficially serrated adenoma components.
Immunohistochemistry
Immunohistochemical analyses were performed on formalin-fixed paraffin-embedded specimens. Ten goblet cell-rich hyperplastic polyps and 10 low-grade tubular adenomas were also examined for comparison. All goblet cell-rich hyperplastic polyps had KRAS mutations, whereas the tubular adenomas had APC mutations [8], indicating that these lesions have prototypical genetic features. Antigen retrieval was performed by autoclaving the samples in 10 mM citrate buffer (pH 6.0) for 10 min. The following primary antibodies were used: anti-CK20 (Ks 20.8; 1:50; Dako, Glostrup, Denmark), anti-Ki-67 (MIB-1; 1:100; Dako), anti-MYC (Y69; 1:200; Abcam, Cambridge, UK), anti-β-catenin (14; 1:200; BD Biosciences, Franklin Lakes, NJ, USA), and anti-MLH1 (ES05; 1:200; Dako). An automated stainer (Dako) was used in accordance with the manufacturer’s protocol. ChemMate EnVision (Dako) methods were used for detection. For β-catenin staining, nuclear expression in <10%, 10–50%, or >50% of cells was classified as −, +, or ++, respectively [8]. For MLH1 staining, lesions devoid of nuclear staining were considered MLH1-expression-deficient, using lymphocytes and endothelial cells as internal positive controls.
DNA and RNA extraction
Deparaffinized, 10-µm-thick sections from each paraffin block were microdissected under a microscope using sterilized toothpicks in order to enrich for tumor content. Five goblet cell-rich hyperplastic polyps and five tubular adenomas were also analyzed for comparison. In superficially serrated adenoma-associated traditional serrated adenomas, the superficially serrated adenoma and traditional serrated adenoma components were separately dissected. The microdissected samples were subjected to DNA and RNA extraction using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) and RNeasy FFPE Kit (Qiagen), respectively.
Next-generation and Sanger sequencing
Next-generation sequencing targeting frequently mutated regions of KRAS, NRAS, BRAF, CTNNB1, APC, RNF43, and GNAS was performed as described previously [17]. All of the detected mutations were verified by Sanger sequencing. The mutation analysis of superficially serrated adenoma-associated traditional serrated adenomas was performed by Sanger sequencing as described previously [16].
Conventional and quantitative RT-PCR
Following reverse-transcription (RT) reactions, complementary DNA sample quality was assessed by PCR amplification of a 220-bp fragment of ACTB [8]. RT-PCR targeting known RSPO fusions, including EIF3E-RSPO2 and PTPRK-RSPO3, was performed as described previously [8]. Quantitative PCR for RSPO2 and RSPO3 was performed using KOD SYBR qPCR Mix (TOYOBO, Tokyo, Japan). RSPO2 and RSPO3 expression levels were determined using GUSB as a standard and compared with four samples from normal colon mucosa. Expression levels threefold higher than those seen in the normal colon mucosa were regarded as overexpression.
Statistical analysis
Fisher’s exact test and Welch’s t test were used to analyze categorical variables and continuous variable, respectively. P values <0.05 were considered to indicate statistical significance.
Results
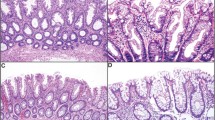
Twenty superficially serrated adenomas were obtained from 11 female and 9 male patients who were aged 62–87 years (Table 1). Eighteen lesions (90%) were located in the sigmoid colon or rectum. They were 2–20 mm in size (median, 5 mm). Endoscopically, they were mostly small sessile polyps, but larger lesions appeared flat/plaque-like (Fig. 1). Histologically, superficially serrated adenomas primarily consisted of straight adenomatous glands but also showed serration in the superficial layer (Fig. 2a–e). The tumor cells were columnar and had uniform basal elongated nuclei (Fig. 2f). The overlying epithelium showed a tufted appearance but did not have the abundant eosinophilic cytoplasm seen in traditional serrated adenomas. None of the lesions showed a villous growth pattern. The bottom of the crypts was generally straight and lacked sessile serrated adenoma/polyp-like features, but larger lesions had some dilated glands. None of the lesions were associated with other types of polyps, such as hyperplastic polyps or sessile serrated adenoma/polyps.
Histological features of superficially serrated adenomas. a, b Low-power images of polyps showing proliferation of adenomatous glands and superficial serration. c Abrupt transition of the neoplastic glands and normal mucosa at the periphery of the lesion. d Tufting of the overlying epithelium. e Straight tumor glands lacking serration in the middle to bottom layer. f Glands lined by columnar epithelium with uniform elongated basal nuclei
We performed comparative immunohistochemical analyses of superficially serrated adenomas to normal colon mucosa, goblet cell-rich hyperplastic polyps, and tubular adenomas (Fig. 3). Since lesions of each type showed similar staining results, representative findings have been presented. CK20 was expressed in the upper layer of goblet cell-rich hyperplastic polyps and superficially serrated adenomas, similar to normal mucosa, indicating preservation of surface differentiation (Fig. 3b, f, j) [18, 19]. In contrast, tubular adenomas showed minimal to weak CK20 staining throughout the crypts (Fig. 3n). Ki-67 expression was localized to the bottom of the crypts in both normal mucosa and goblet cell-rich hyperplastic polyps (Fig. 3c, g). In superficially serrated adenomas, the Ki-67-positive cell compartment extended to the upper half of the crypts (Fig. 3k), while tubular adenomas showed expression predominantly in the upper layer (Fig. 3o). The distribution pattern of MYC-positive cells appeared similar to that of the proliferating cells in each type of lesion (Fig. 3d, h, l, p). Nuclear β-catenin expression was absent in both normal mucosa and goblet cell-rich hyperplastic polyps (Fig. 4a, b) while appearing either focal or diffuse in superficially serrated adenomas and diffuse in tubular adenomas (Fig. 4c, d).
Comparative immunohistochemical analysis. a–d Normal mucosa. e–h Goblet cell-rich hyperplastic polyp. i–l Superficially serrated adenoma. m–p Tubular adenoma. CK20 is expressed in the upper layer of normal mucosa, goblet cell-rich hyperplastic polyp, and superficially serrated adenoma (b, f, j). Tubular adenoma showing diffuse weak CK20 expression (n). Ki-67 and MYC expression is localized to the bottom layer in normal mucosa and goblet cell-rich hyperplastic polyp (c, d, g, h), whereas distributed in the middle to lower layers in superficially serrated adenoma (k, l) and in the upper layer in tubular adenoma (o, p)
β-Catenin expression in normal mucosa, a goblet cell-rich hyperplastic polyp, a superficially serrated adenoma, and a tubular adenoma. a, b Epithelial cells of normal mucosa (a) and a goblet cell-rich hyperplastic polyp (b) show exclusively membranous β-catenin expression. c A superficially serrated adenoma shows moderate nuclear β-catenin staining in addition to membranous staining. d A tubular adenoma shows diffuse strong nuclear β-catenin accumulation
Next-generation sequencing identified activating KRAS mutations in 19 of 20 superficially serrated adenomas and a BRAF mutation in the one remaining (Table 1). Two lesions had truncating APC mutations and one lesion had a GNAS mutation. These results were confirmed by Sanger sequencing. NRAS, CTNNB1, and RNF43 mutations were absent. RT-PCR identified PTPRK-RSPO3 fusions in 11 lesions (Fig. 5a, b). Quantitative PCR revealed overexpression of RSPO3 exons 3 and 4 in 16 superficially serrated adenomas (Fig. 5c), including all the 11 lesions containing PTPRK-RSPO3 fusions. RSPO3 exons 1 and 2 overexpression, suggestive of overexpression of full-length RSPO3, was observed in five lesions. Two lesions that lacked RSPO3 overexpression showed overexpression of RSPO2 exons 1 and 2, and 3 and 4, indicating overexpression of full-length RSPO2. Collectively, 18 superficially serrated adenomas showed RSPO fusions and/or overexpression (Table 1). RSPO fusions/overexpression was mutually exclusive with APC mutations. Although we histologically reexamined the APC-mutated lesions, these were basically indistinguishable from the other superficially serrated adenomas, whereas one lesion (SuSA20) showed slightly lesser superficial serration. Overexpression of RSPO2 and RSPO3 was absent in all goblet cell-rich hyperplastic polyps and tubular adenomas.
Gene expression analyses of RSPO in superficially serrated adenomas. a RT-PCR amplifications of RSPO fusions. PTPRK exon 1-RSPO3 and PTPRK exon 7-RSPO3 fusions were detected in two (SuSA13 and 19) and four (SuSA12, 14, 15, and 17) superficially serrated adenomas, respectively. EIF3E-RSPO2 fusions were not detected in any samples. ACTB served as a positive control. b Sanger sequencing of RSPO fusion junctions and KRAS. The PTPRK exon 7-RSPO3 fusion product was sequenced using a reverse primer. c RSPO expression analysis in superficially serrated adenomas. Quantitative RT-PCR analysis of RSPO2 exons 1 and 2, and exon 3 and 4 (left), and RSPO3 exons 1 and 2, and exons 3 and 4 (right). Expression levels are indicated relative to normal colon mucosa. Five goblet cell-rich hyperplastic polyps (HP) and five tubular adenomas (TA) were also analyzed for comparison
Since concurrent RSPO fusions/overexpression and KRAS mutations are common genetic features of traditional serrated adenomas [8, 16], we histologically reviewed 129 traditional serrated adenomas and identified 15 (12%) that were associated with a superficially serrated adenoma component (Table 2 and Supplementary Table 1). The patients with superficially serrated adenoma-associated traditional serrated adenomas were 44–80 years of age and predominantly female in contrast to those without superficially serrated adenoma components, which occur more frequently in male patients. All of the superficially serrated adenoma-associated traditional serrated adenomas were located in the sigmoid colon or rectum. The traditional serrated adenoma and superficially serrated adenoma components were usually recognizable as polypoid and sessile areas, respectively, in both endoscopic and histological examination (Fig. 6) and were histologically well-demarcated. Superficially serrated adenoma-associated traditional serrated adenomas showed more prominent ectopic crypt foci and less slit-like serration and were more likely to have high-grade components compared with those without superficially serrated adenoma components. The superficially serrated adenomas associated with traditional serrated adenomas showed immunohistochemical features similar to solitary superficially serrated adenomas, including CK20, Ki-67, and MYC expression patterns (Supplementary Figure 1). Focal or diffuse nuclear β-catenin expression was observed in 11 of 15 lesions (73%). The extent of β-catenin expression was similar between the two components in most lesions but more extensive in the traditional serrated adenoma component of some. MLH1 expression was consistently retained in both components.
A traditional serrated adenoma associated with a superficially serrated adenoma. a Endoscopic image showing a protruding polyp within a slightly elevated sessile lesion (arrowheads). b Panoramic view showing a polypoid component with lobulation and a sessile component (arrowheads). c Traditional serrated adenoma component exhibiting classical histological features such as villous architecture and ectopic crypt formation. d, e Superficially serrated adenoma component. Non-serrated adenomatous glands occupy the middle to lower layer, whereas the upper layer shows serration
Referring to the results of our previous mutation and expression analyses [8, 16], we noted that all superficially serrated adenoma-associated traditional serrated adenomas had KRAS mutations and all but one lesion (93%) showed RSPO fusions and/or overexpression. One lesion (7%) had a GNAS mutation. We then analyzed the presence of these mutations and RSPO fusions/overexpression in each component. The results showed that all the mutation and RSPO expression statuses were concordant between the two components of each lesion (Fig. 7 and Supplementary Table 1).
RSPO expression in superficially serrated adenoma-associated traditional serrated adenomas. a–c RT-PCRs targeting the PTPRK exon 1-RSPO3 (a), PTPRK exon 6-RSPO3 (b), and PTPRK exon 7-RSPO3 (c) fusions of superficially serrated adenoma and traditional serrated adenoma components of superficially serrated adenoma-associated traditional serrated adenomas. ACTB served as a positive control. d Sanger sequencing results for the RSPO fusion junctions and KRAS. The PTPRK exon 7-RSPO3 fusion product was sequenced using a reverse primer. e, f Quantitative RT-PCR analysis of the RSPO2 exons 1 and 2 and exons 3 and 4 (e) and RSPO3 exons 3 and 4 (f) junctions of the superficially serrated adenoma and traditional serrated adenoma components of superficially serrated adenoma-associated traditional serrated adenomas. Expression levels are indicated relative to normal colon mucosa. Notice that RSPO3 overexpression was detected in both components in all the lesions with RSPO fusions and/or overexpression. T traditional serrated adenoma, S superficially serrated adenoma, N normal mucosa
Discussion
Our histological and immunohistochemical analyses demonstrated that superficially serrated adenomas share several common features with low-grade tubular adenomas. Superficially serrated adenomas primarily comprise straight adenomatous glands and exhibit nuclear accumulation of β-catenin and overexpression of MYC, suggestive of WNT pathway activation [20]. Unlike tubular adenomas, however, proliferative cells localize to the middle and lower layers of the mucosa while the superficial epithelium exhibits serration and CK20 expression; these findings more closely resemble those of goblet cell-rich hyperplastic polyps. We may need to consider sessile serrated adenoma/polyps with dysplasia while formulating the differential diagnosis. However, superficially serrated adenomas lack non-dysplastic sessile serrated adenoma/polyp components and are mostly localized to the distal colon [3, 14]. Collectively, superficially serrated adenomas exhibit mixed adenomatous and serrated features and are distinct from currently recognized categories of colorectal polyps.
Mutation and gene expression analyses indicated that superficially serrated adenomas are fairly genetically homogeneous, as most harbor KRAS mutations together with RSPO fusions and/or overexpression. Most serrated lesions exhibit either KRAS or BRAF mutations that lead to mitogen-activated protein kinase (MAPK) pathway activation, although the frequencies vary. For example, KRAS mutations are reportedly common in goblet cell-rich hyperplastic polyps and traditional serrated adenomas but rare in microvesicular hyperplastic polyps and sessile serrated adenoma/polyps [4, 8]. The WNT pathway gene mutation profiles are also distinct among the different polyp types. RSPO fusions, which activate WNT signaling by inducing the overexpression of R-spondin and downregulation of RNF43 [21], are present in one-third of traditional serrated adenomas but absent in conventional adenomas [8, 16]. A previous study by our group also identified the overexpression of full-length RSPO as an alternative mechanism of WNT activation in a subset of traditional serrated adenomas [16]. APC mutations, which were detected in two superficially serrated adenomas, are present in the majority of conventional adenomas but infrequently detected in serrated lesions. Therefore, the concurrent presence of KRAS mutations and RSPO fusions/overexpression in most superficially serrated adenomas supports the classification of superficially serrated adenoma as a serrated lesion.
We also noted an association with a superficially serrated adenoma component in a subset of traditional serrated adenomas. Strikingly, all but one superficially serrated adenoma-associated traditional serrated adenoma exhibited KRAS mutations and RSPO fusions and/or overexpression. Furthermore, the superficially serrated adenoma and traditional serrated adenoma components of these lesions consistently exhibited concordant KRAS mutation and RSPO expression statuses. Whereas previous studies demonstrated that KRAS-mutated traditional serrated adenomas are rarely associated with precursor polyps, superficially serrated adenomas may be an important precursor of KRAS-mutated traditional serrated adenomas. Simultaneously, our observations imply that the combination of a KRAS mutation and an RSPO fusion is not sufficient to induce the characteristic protuberant morphology of a traditional serrated adenoma and that additional unidentified factors are required for the development of traditional serrated adenomas.
Traditional serrated adenomas, particularly those with BRAF mutations, are often associated with hyperplastic polyps or sessile serrated adenoma/polyps [13, 22,23,24,25,26,27]; however, only a few studies have recognized the association of a discrete adenomatous component with traditional serrated adenomas [22, 25, 27]. Notably, one study described the association of a “tubular adenoma-like lesion,” which may be consistent with superficially serrated adenoma, in 10 of 107 traditional serrated adenomas [25]. These tubular adenoma-like lesions reportedly comprised straight adenomatous glands, with Ki-67-positive proliferating cells localized to the lower two-thirds of the crypts. Furthermore, a magnified endoscopic examination of these lesions revealed a hyperplastic polyp-like surface pattern. Although this study did not provide the detailed clinicopathological or molecular features of traditional serrated adenomas associated with a tubular adenoma-like lesion, it appears to suggest the common presence of superficially serrated adenoma-associated traditional serrated adenomas in different case series.
It may be tempting to regard superficially serrated adenoma as a variant of traditional serrated adenoma, based on their frequent coexistence and overlapping mutation profiles. However, superficially serrated adenomas were mostly small sessile polyps that lacked the classical morphological features of traditional serrated adenomas, including the typical cytology, ectopic crypt formation, and slit-like serration [13]. Thus, it is difficult to identify a morphological link between solitary superficially serrated adenomas and traditional serrated adenomas. Indeed, most superficially serrated adenomas examined in the present study were originally diagnosed as low-grade tubular adenomas. Additionally, when superficially serrated adenomas were observed in association with traditional serrated adenomas, these two components were sharply demarcated. Moreover, superficially serrated adenomas exhibit a rather uniform genetic profile, unlike traditional serrated adenomas, which harbor diverse sets of WNT and MAPK pathway gene mutations [8].
In conclusion, the present study has characterized the morphological and molecular features of colorectal superficially serrated adenoma. The concurrent presence of KRAS mutations and RSPO fusions/overexpression is a common molecular feature of superficially serrated adenomas and suggests that these can be classified as serrated lesions, despite their predominantly adenomatous morphology. Although superficially serrated adenomas appear as solitary polyps, they may be associated with traditional serrated adenomas. Given the frequent associations and overlapping mutation profiles of superficially serrated adenomas and traditional serrated adenomas, superficially serrated adenomas might be an important precursor to KRAS-mutated traditional serrated adenomas. Further studies are needed to establish superficially serrated adenoma as a distinct histological subtype of serrated lesions; however, we believe that the recognition of this entity contributes to the appropriate histological classification of colorectal polyps and a better understanding of colorectal tumorigenesis.
References
Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–30.
Hamilton SR, Bosman FT, Boffetta P, et al. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press; 2010. p. 134–46.
Snover DC, Ahnen DJ, Burt RW, et al. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press; 2010. p. 160–5.
Bettington M, Walker N, Clouston A, et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–86.
Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–100.
Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–7.
Yang S, Farraye FA, Mack C, et al. BRAF and KRAS mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol. 2004;28:1452–9.
Sekine S, Yamashita S, Tanabe T, et al. Frequent PTPRK-RSPO3 fusions and RNF43 mutations in colorectal traditional serrated adenoma. J Pathol. 2016;239:133–8.
Tsai JH, Liau JY, Yuan CT, et al. RNF43 is an early and specific mutated gene in the serrated pathway, with increased frequency in traditional serrated adenoma and its associated malignancy. Am J Surg Pathol. 2016;40:1352–9.
Hashimoto T, Yamashita S, Yoshida H, et al. WNT pathway gene mutations are associated with the presence of dysplasia in colorectal sessile serrated adenoma/polyps. Am J Surg Pathol. 2017;41:1188–97.
Sheridan TB, Fenton H, Lewin MR, et al. Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions “caught in the act”. Am J Clin Pathol. 2006;126:564–71.
Tsai JH, Liau JY, Lin YL, et al. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol. 2014;27:1375–85.
Bettington ML, Walker NI, Rosty C, et al. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol. 2015;28:414–27.
Bettington M, Walker N, Rosty C, et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97–106.
Bettington M, Walker N, Rosty C, et al. Serrated tubulovillous adenoma of the large intestine. Histopathology. 2016;68:578–87.
Sekine S, Ogawa R, Hashimoto T, et al. Comprehensive characterization of RSPO fusions in colorectal traditional serrated adenomas. Histopathology. 2017;71:601–9.
Sekine S, Mori T, Ogawa R, et al. Mismatch repair deficiency commonly precedes adenoma formation in Lynch syndrome-associated colorectal tumorigenesis. Mod Pathol. 2017;30:1144–51.
Davenport A, Hale RJ, Hunt CR, et al. Expression of Ki-67 and cytokeratin 20 in hyperplastic polyps of the colorectum. J Clin Pathol. 2003;56:200–4.
Chan CW, Wong NA, Liu Y, et al. Gastrointestinal differentiation marker cytokeratin 20 is regulated by homeobox gene CDX1. Proc Natl Acad Sci USA. 2009;106:1936–41.
Sansom OJ, Meniel VS, Muncan V, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–9.
de Lau W, Peng WC, Gros P, et al. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28:305–16.
Iino H, Jass JR, Simms LA, et al. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol. 1999;52:5–9.
Lee EJ, Choi C, Park CK, et al. Tracing origin of serrated adenomas with BRAF and KRAS mutations. Virchows Arch. 2005;447:597–602.
Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol. 2008;32:21–29.
Kim MJ, Lee EJ, Suh JP, et al. Traditional serrated adenoma of the colorectum: clinicopathologic implications and endoscopic findings of the precursor lesions. Am J Clin Pathol. 2013;140:898–911.
Wiland HO 4th, Shadrach B, Allende D, et al. Morphologic and molecular characterization of traditional serrated adenomas of the distal colon and rectum. Am J Surg Pathol. 2014;38:1290–7.
Chetty R, Hafezi-Bakhtiari S, Serra S, et al. Traditional serrated adenomas (TSAs) admixed with other serrated (so-called precursor) polyps and conventional adenomas: a frequent occurrence. J Clin Pathol. 2015;68:270–3.
Acknowledgements
We thank Ms. Sachiko Miura, Ms. Toshiko Sakaguchi, Ms. Chizu Kina, and Ms. Yuka Nakamura for their skillful technical assistance. This work was supported by JSPS KAKENHI grant number JP16K19097.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hashimoto, T., Tanaka, Y., Ogawa, R. et al. Superficially serrated adenoma: a proposal for a novel subtype of colorectal serrated lesion. Mod Pathol 31, 1588–1598 (2018). https://doi.org/10.1038/s41379-018-0069-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-018-0069-8
This article is cited by
-
Sessile serrated lesions with dysplasia: is it possible to nip them in the bud?
Journal of Gastroenterology (2023)
-
Traditional serrated adenoma has two distinct genetic pathways for molecular tumorigenesis with potential neoplastic progression
Journal of Gastroenterology (2020)
-
Clinicopathological and molecular correlations in traditional serrated adenoma
Journal of Gastroenterology (2020)
-
An update on the morphology and molecular pathology of serrated colorectal polyps and associated carcinomas
Modern Pathology (2019)
-
Identification of a novel PRR15L-RSPO2 fusion transcript in a sigmoid colon cancer derived from superficially serrated adenoma
Virchows Archiv (2019)